Abstract
Objective
The inflammatory response and the presence of macrophages are reported to be necessary for proper muscle regeneration. However, our understanding of the molecular mechanisms governing how macrophages signal to promote muscle regeneration is incomplete.
Methods and results
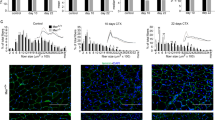
Here we conditionally deleted Wls, which is required for Wnt secretion, from macrophages and examined the impact on endothelial permeability following muscle injury. The expression of Wnt ligands and Wls was increased in the tibialis anterior (TA) of mice 2 days following BaCl2 injury. Loss of macrophage Wls inhibited the loss of endothelial barrier function, as measured by transendothelial resistance and Evans blue dye permeability assays. Interestingly, the blockade in endothelial permeability correlated with reduced VEGF levels and pretreatment of wild type endothelial cells with a VEGFR2 blocking antibody was sufficient to reduce endothelial permeability induced by stimulated macrophage supernatant. We also found that macrophage Wls-null TAs had myocytes with reduced cross-sectional area 7 day post-injury suggesting a delay in muscle regeneration.
Conclusion
Our results indicate that macrophage-derived Wnt signaling increases endothelial permeability in a VEGF-dependent fashion following muscle injury. Our findings implicate macrophages as a primary source of Wnt ligands following muscle injury and highlight the Wnt pathway as a therapeutic target following injury.





Similar content being viewed by others
References
Brown LF, Yeo K, Berse B, Yeo T, Senger R, Dvorak HF, Van De Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176(5):1375–9.
Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–58.
Muller WA. Mechanisms of leukocyte transendothelial migration. Ann Rev Path. 2011;6:323–44.
Koh TJ, Byer SC, Pucci AM, Sisson TH. Mice deficient in plasminogen activator inhibitor-1 have improved skeletal muscle regeneration. Am J Physiol. 2005;289(1):C217–C223223.
Lluis FJ, Roma M, Suelves M, Parra M, Aniorte G, Gallardo E, Illa I, Rodriguez L, Hughes SM, Carmeliet P, Roig M, Munoz-Canoves P. Urokinase-dependent plasminogen activation is required for efficient skeletal muscle regeneration in vivo. Blood. 2001;97(6):1703–11.
Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Discord. 1999;9(2):72–80.
Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, Shireman PK. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R832–R842842.
Hsieh PL, Rybalko V, Baker AB, Suggs LJ, Farrar RP. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia. J Vasc Surg. 2018;67(6):1908–20.
Teixeira CF, Zamuner SR, Zuliani JP, Fernandes CM, Cruz-Hofling MA, Fernandes I, Chaves F, Gutierrez JM. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve. 2003;28(4):449–59.
Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25(1):358–69.
Latroche C, Weiss-Gayet M, Muller L, Gitiaux C, Leblanc P, Liot S, Ben-Larbi S, Abou-Khalil R, Verger N, Bardot P, Magnan M, Chretien F, Mounier R, Germain S, Chazaud B. Coupling between myogenesis and angiogenesis during skeletal muscle regeneration is stimulated by restorative macrophages. Stem Cell Rep. 2017;9(6):2018–33.
Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–22.
Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125(3):523.
Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133(24):4901–11.
Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci. 2010;107(9):4194–9.
Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2(1):50–9.
LeGrand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4(6):535–47.
Polesskaya A, Seale P, Rudnicki MA. Wnt Signaling induces the myogenic specification of resident CD45+ stem cells during muscle regeneration. Cell. 2003;113(7):841–52.
Zhao P, Hoffman EP. Embryonic Myogenesis pathways in muscle regeneration. Dev Dyn. 2004;229(2):380–92.
vonMaltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22(11):602–9.
Carpenter AC, Rao S, Wells JM, Campbell K, Lang R. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–8.
Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distince, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–655.
Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, William BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474(7352):511–5.
Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010. https://doi.org/10.3791/1488.
Wang Y, Alexander JS. Analysis of endothelial barrier function in vitro. Methods Mol Biol. 2011;763:253–64.
Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thepenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon J, Tajbakhsh, Rocheteau P, Chretien F. Comparative study of injury models for studying muscle regeneration in mice. Plos One. 2016; https://doi.org/10.1371/journal.pone.0147198.
M. Radu M, J. Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp. 2013; https://doi.org/10.3791/50062.
Hedgepeth CM, Conrad LJ, Zhang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185(1):82–91.
Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. β-Catenin regulates vascular endothelial growth factor expression in colon cancer. Can Res. 2003;63:3145–53.
Weis S, Cheresh D. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. https://doi.org/10.1038/nature03987.
Tojais NF, Peghaire C, Franzl N, Larrieu-Lahargue F, Jaspard B, Reynaud A, Moreau C, Couffinhal T, Duplàa C, Dufourcq P. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovas Res. 2014;103(2):291–303.
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kou C, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. PNAS. 2009;106(2):641–6.
Liebner S, Corado M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J of Cell Biol. 2008;183(3):409–417.
Laksitorini MD, Yathindranath Y, Xiong W, Homback-Klonisch S, Miller DW. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci Rep. 2018;9:19718.
Lim RG, Quan C, Reyes-Ortiz AM, Lutz SE, Kedaigle AJ, Gipson TA, Wu J, Vatine GD, Stocksdale J, Casale M, Svedsen CN, Fraenkel E, Housman DE, Agalliu D, Thompson LM. Huntington’s Disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep. 2017;19:1365–77.
Skaria T, Bachli E, Schoedon G. Wnt5A/Ryk signaling affects barrier function in human vascular endothelial cells. Cell Adh Migr. 2017;11(1):24–38.
Kim J, Kim J, Kim DW, Ha Y, Ihm MH, Kim H, Song K, Lee I. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–82.
Gavard J. Endothelial permeability and VE-cadherin. Cell Ad Mig. 2013;7(6):465–71.
Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol. 1995;54:121–8.
Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Mazzoleni G, Vittiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurologl Sci. 2002;23:189–94.
Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2-/- mice during impaired skeletal muscle regeneration. Am J Phys Reg Integr Comput Phys. 2007;293(2):R651–R661661.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tusavitz, S., Keoonela, S., Kalkstein, M. et al. Macrophage-derived Wnt signaling increases endothelial permeability during skeletal muscle injury. Inflamm. Res. 69, 1235–1244 (2020). https://doi.org/10.1007/s00011-020-01397-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01397-z