Abstract
Taraxacum pieninicum Pawł. is listed as critically endangered species, for which currently applied protection methods are insufficient. The aim of this study was to investigate the possibility of T. pieninicum storage in the form of synthetic seeds under slow-growth conditions in combination with ABA treatment, as one of the ex situ protection methods of this species. The obtained results indicated that darkness was much more favorable condition for synseed storage and did not generate additional stress during cold exposure in contrast to the light conditions. The preculture of shoot tips on the medium supplemented with ABA led to a decrease in the shoots proliferation rate and inhibition of their growth. ABA clearly inhibited growth of the encapsulated shoot tips also during cold storage. Biochemical parameters showed that ABA effectively reduced the negative effect of the cold stress, what was found on the basis of analyzes of H2O2 and TBARS levels in the stored material. Moreover, synseeds stored under light conditions and treated with ABA exhibited decreased level of endogenous jasmonic acid what indicated interaction between those two phytohormones at a low temperature. The study also demonstrated that in vitro culture, cold storage and ABA treatment had no effect on the flowering process of this species after acclimatization to ex vitro conditions.
Key message
The aim of this research was to assess ABA effect on post-storage regrowth and the stress factors level in plants obtained after long-term storage of Taraxacum pieninicum synthetic seeds.
Similar content being viewed by others
Introduction
Taraxacum pieninicum belongs to the Asteraceae family and is a stenochoric species, occurring in only one site in the Central Pieniny in the Western Carpathians (Poland) (Piękoś-Mirkowa and Mirek 2009). This species is considered to be probably the oldest endemic species in Pieniny Mts. from the Paleogene (Puchalski et al. 2014). Creating collections in botanical garden as a method of its protection is not very effective due to the difficulty of keeping it in these areas. As the only species of the Taraxacum genus, it does not produce sesquiterpene lactones, such as taraxic acid and triterpenes, commonly referred to as taraxacin in older literature, which gives the leaf a bitter taste (Schütz et al. 2006). Lack of biochemical protection means that it is eagerly eaten by various herbivore species. In addition, seeds lose their viability (Honek et al. 2005), therefore the in vitro method was considered appropriate for protection of this species. An efficient regeneration system for T. pieninicum has been developed (Trejgell et al. 2013), nevertheless maintaining cultivars in in vitro conditions is costly and carries the risk of contamination (Pence 2010; Cruz-Cruz et al. 2013). For these reasons optimization of the slow growth conditions is highly desirable to maximize time between passages. Lowering the temperature of T. pieninicum shoot tips culture allowed its storage for several months (Kamińska et al. 2018a), although increased survival of the explants was noted after encapsulation (Kamińska et al. 2018b). The possibility of extending the storage time of encapsulated explants by using abscisic acid as a growth retardant was evaluated in this paper.
Growth retardants are synthetic or natural chemical compounds that can cause structural changes or affect plant life processes by direct effect on their hormonal balance. Synthetic retardants that inhibit plant elongation growth are usually gibberellin antagonists that affect their metabolism (Espindula et al. 2009). Natural growth retardants include phytohormones, as abscisic acid (ABA) and jasmonic acid (JA), which show diverse physiological activity (Heinrich et al. 2012).
Endogenous and exogenous ABA is considered as auxins and gibberellins antagonist, leading to inhibition of cell division and elongation growth (Humplík et al. 2017; Lorrai et al. 2018). In conditions of drought, salinity or low temperature stress, this compound promotes closing stomata to reduce water loss by transpiration (Kim et al. 2010), affects the change in the morphology of the root system (Sah et al. 2016) and activates many genes encoding enzymes responsible for the biosynthesis of osmoregulatory substances and LEA-type proteins (Fujita et al. 2011).
The greatest importance in the reaction of plants to abiotic stress is attributed to abscisic acid. The role of ABA in acclimatization to cold is confirmed by increased freezing tolerance in plants growing at room temperature treated with ABA (Palva et al. 2002). In addition, the involvement of ABA in the activation of genes expression related to cold acclimatization has been documented many times. In our previous studies, we have shown the possibilities of storing T. pieninicum synseeds and the usage of jasmonic acid as a growth retardant (Kamińska et al. 2018a, b). The aim of this research was to assess the effect of abscisic acid on the condition of plants obtained after long-term storage of Taraxacum pieninicum synthetic seeds. Verification of the ABA influence on T. pieninicum stored explants was based on the alterations in post-storage regrowth, biochemical stress factors, endogenous ABA and JA content.
Materials and methods
Plant material and culture conditions
Seeds of T. pieninicum were obtained from the collection of the Polish Academy of Sciences Botanical Garden—Center for Biological Diversity Conservation in Powsin. The seeds were sterilized with 70% ethanol for 30 s, followed by 20% sodium hypochlorite solution for 20 min. After this time, the seeds were washed four times with sterile distilled water and were transferred onto MS medium (Murashige and Skoog 1962) without plant growth regulators. MS medium was supplemented with 3% sucrose, solidified with 0.8% agar and adjusted to pH 5.75–5.8 prior to autoclaving for 20 min at 121 °C.
Shoot tips of 12-day-old seedlings were isolated and transferred onto proliferation medium to induce the process of development of axillary buds and their proliferation. MS medium (see above) supplemented with 1.1 μM benzylaminopurine (BAP) and 0.14 μM naphthyl-1-acetic acid (NAA) was used for shoots multiplication (MSBAP). Cultures were kept in a growth room at 26 ± 1 °C, under continuous light with a quantum irradiation intensity of 80 μmol m−2 s−1 (optimal growth conditions). After 4 weeks of culture, obtained axillary buds were isolated and transferred on fresh growth medium with the same composition.
Synthetic seeds production
Individual shoot tips after isolation from the culture of T. pieninicum axillary shoots were placed on a Petri dish containing 3% sodium alginate dissolved in liquid MS medium. A single explant covered with alginate solution was added dropwise to a 100 mM calcium chloride solution. Polymerization of alginate was carried out for 25 min with gentle stirring of the calcium chloride solution. Using sterile strainer, the calcium chloride solution was removed and the obtained synthetic seeds were rinsed with sterile distilled water.
Abscisic acid treatment
Two methods of ABA exposure were used: a four-week shoots preculture in the optimal growth conditions on MSBAP medium supplemented with ABA preceding encapsulation (further indication in the paper—preculture) and addition of ABA to the alginate solution during shoot tips encapsulation (inside). For both experiments four concentrations of ABA were used: 19 μM, 38 μM, 57 μM and 76 μM. After preculture proliferation rate (number of shoots per explant) and visual evaluation of the shoots growth were additionally assessed.
Cold storage conditions
Synseeds were placed on 50 mL MS medium without plant growth regulators in polycarbonate culture boxes (Magenta™ vessel, Sigma-Aldrich). Storage was carried out for 3, 6 or 9 months at 4 ± 1 °C in conditions of continuous light with a reduced quantum irradiation intensity to 40 μmol m−2 s−1 or in the dark. Synseeds non-treated with ABA were used as a control.
Post-storage regrowth
Directly after cold-storage a visual evaluation of the shoots and roots length was performed, then alginate coat was gently removed, leaves were collected for biochemical analyzes and isolated shoot tips were transferred onto MSBAP medium to the optimal growth conditions. The proliferation rate was assessed after 4 weeks of 2 subcultures. For rooting shoots after 2nd subculture were transferred onto MS medium without plant growth regulators for 4 weeks. Rooting ability was investigated in terms of percent shoots able to form roots, number of roots per shoot and root length. Roots were gently washed with sterile distilled water and then plantlets were transferred to pots containing sterile vermiculite and sand (1:1 v/v). The pots were covered with a transparent lid to maintain humidity (60–70%). Plantlets grown at 23 ± 1 °C under white fluorescent light (80 μmol m−2 s−1) for 4 weeks after which they were transferred to the pots containing sterile soil and then were transferred to the field conditions. Survival rate and ability to flower of the plantlets were noted in the next year after acclimatization (April/May).
Biochemical analyzes
Directly after cold-storage leaves of stored explants were analyzed in terms of chlorophyll, H2O2, TBARS, soluble sugars, free proline and endogenous phytohormone (ABA, JA) content. Leaves from stored explants non-treated with ABA were used as a control. Additional non-stored plant tissue from optimal growth conditions was used for phytohormone analyzes. Chlorophyll, H2O2, soluble sugars and proline content was also determined in leaves after 4-weeks preculture on medium supplemented with ABA under optimal growth conditions. Chlorophyll was extracted from 20 mg of plant tissue in 96% ethanol and determined by the method of Nair and Chung (2015). To determine the total chlorophyll content (a and b) the formula developed by Lichtenthaler (1987) was used:
Hydrogen peroxide was determined according to Velikova et al. (2000). For extraction 100–200 mg of leaves was used and determination of H2O2 was based on the iodide-reduction method with 1 M KI solution. TBARS level was estimated following the protocol described by Song et al. (2011). Sample for this analysis contained approximately 50 mg of leaves. Free proline was determined spectrophotometrically using method based on a reaction with ninhydrin (Bates et al. 1973). Extraction and determination of total soluble sugars was carried out by the phenol–sulfuric acid method (PSA) described by Dubois et al. (1956). Glucose was used to prepare standard curve. Endogenous level of abscisic acid and jasmonic acid was determined by using mass spectrometry combined with liquid chromatography (LC–MS). Analysis was carried out for explants stored for 9 months on MS medium without ABA and treated with this phytohormone at concentrations of 38–76 μM. Sample contained 50–100 mg of leaves homogenized in liquid nitrogen (LN). For the extraction of phytohormones 80% methanol (v/v) was added. In this step also small amount of antioxidant butylhydroxytoluol (BHT), 5 ng of deuterated ABA (d6ABA) and 10 ng deuterated JA (d5JA) as internal standards were added. The mixture was shaken overnight (at least 18 h). After incubation, the samples were centrifuged and methanol was evaporated. To remove chlorophyll, the samples were acidified to pH 2 by the addition of hydrochloric acid. After centrifuged the extracts, the supernatant was subjected to solid phase extraction (SPE) using silica packed columns (Discovery® DSC-18 SPE Tube). The columns were activated with 100% methanol and conditioned with formic acid. The applied samples were purified with 1 M formic acid and a solution of 1 M formic acid in 20% methanol (v/v). Elution was performed using 80% methanol (v/v). Before analysis samples were concentrated 7.5 times and centrifuged. For phytohormones determination the LCMS-8045 tandem mass spectrometry (Shimadzu Corp.) was used. Chromatographic separation was carried out on a Kinetex® 2.6 μm XB-C18 100 Å reverse phase column (150 × 2.1 mm). Water with 0.1% formic acid (v/v) (A) and methanol with 0.1% formic acid (v/v) (B) were used as the mobile phase. The separation was carried out in a linear gradient of 40–90% (v/v) methanol for 7 min at a flow rate of 0.3 mL/min at 30 °C. In mass spectrometry, the samples were subjected to negative electrospray ionization (ESI) and ions were fragmented by collision-induced dissociation (CID). The ionization voltage was − 3 kV. Analysis of individual phytohormones was based on multiple reactions monitoring (MRM).
Data collection and statistical analysis
The results presented in this paper were expressed as the mean ± standard error. Parameters from preculture (proliferation rate), storage (survival) and post-storage regrowth (proliferation rate and rooting ability of the shoots) were evaluated for 16 explants in each variant in three replicates. The leaves of survived explants from each vessel were grounded in LN together and biochemical analyzes were evaluated for 3 samples (1 representative sample from all of the explants stored in 1 vessel). Each biochemical test was performed in 3 biological and 3 technical replicates. The normality of the distribution of obtained results was tested by the Shapiro–Wilk test. In the case of data with non-normal distribution, the significance of differences was determined using non-parametric Mann–Whitney U test. Data with normal distribution and obtained in biochemical analyzes were analyzed using Tukey’s test. In addition, the significance of the impact of the studied factors on the stored plant material and their interactions on the accumulation of selected chemical compounds was analyzed using two- and three-way ANOVA.
Results
Preculture with ABA in optimal conditions
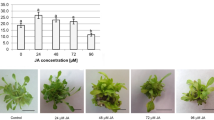
The obtained results indicated that ABA, at all tested concentrations, significantly decreases shoot proliferation rate in comparison to the control (18.9 ± 1.6 shoots per explant). Proliferation rate of ABA-treated shoots ranged from 10.1 ± 1.4 (19 μM ABA) to 3.3 ± 0.4 (76 μM ABA) shoots per explants (Fig. 1a). In addition, it was observed that increasing ABA concentration slightly decreased shoots length (Fig. 1b).
The effect of ABA on T. pieninicum shoot proliferation in the optimal growth conditions: proliferation rate (a) and phenotype of shoots cluster (b) on MS medium supplemented with 1.11 μM BAP and 0.14 μM NAA and different ABA concentration after 4 weeks of the culture. Bar = 1 cm. Means with different letters are significantly different followed by Kruskal–Wallis test at p ≤ 0.05
ABA treatment during cold-storage
Preliminary observation indicated that exposure to ABA reduced growth of the shoots obtained during synseed storage in comparison to the control, both after 3 and 6 months of storage (Figs. S1-S2) and after 9 months, where a particularly significant reduction of shoot length occurred in the presence of 76 μM ABA inside artificial endosperm in both light and dark conditions (Fig. 2). In addition, shoots stored in the dark in all tested ABA variants did not form roots, regardless of the ABA concentration. However, when storing synseeds under light conditions ABA-treated shoots during preculture were able to form roots during cold storage for 6 and 9 months, but their elongation was inhibited (Figs. 2, S1-S2).
Regrowth of the stored explants under optimal conditions
After cold-storage the survival and shoots proliferation ability under the optimal growth conditions were analyzed. After 9 months storage treatment with ABA did not affect explants survival (Fig. 3). Over 75% of synseeds remained viable, regardless of the ABA treatment. Light conditions during storage decreased synseeds viability, although differences to the dark were not statistically significant in ABA-treated explants. Similar results were obtained after 6 months storage. Reduced survival of the synseeds treated with ABA was observed only after 3 months storage particularly under light conditions and when ABA was added inside synseed structure (Fig. S3).
Survival of the encapsulated shoot tips of T. pieninicum after 9 months storage 4 °C. Statistically significant differences assessed using Kruskal–Wallis test at p ≤ 0.05: to the control plants in the same light conditions are denoted *(p < 0.05), **(p < 0.01), ***(p < 0.001); between light conditions are denoted #(p < 0.05), ##(p < 0.01), ###(p < 0.001); between ABA treatment in the same light conditions are denoted ◊(p < 0.05), ◊◊(p < 0.01), ◊◊◊(p < 0.001)
The light conditions during cold-storage had the most significant impact on the post-storage proliferation ability of the explants under optimal growth conditions. Higher proliferation rate was indicated after storage in the dark in comparison to the light conditions, significant differences were noted for most of the used variants. After 9 months of storage ABA-treated synseeds remained comparable proliferation ability to the control stored in the light (7.6 ± 1.2 shoots per explant) and dark (13.7 ± 1.4) conditions (Fig. 4a). The method of ABA exposure was significant mostly after shorter storage time (Fig. S4). Similar relationships were observed after the second post-storage subculture (Fig. 4b). In comparison to the control, prior ABA treatment did not inhibit shoots proliferation ability. The highest proliferation rate in 2nd subculture was noted for shoots obtained from synseeds containing 38 μM ABA inside and stored in the dark for 9 months (23.7 ± 1.8; Fig. 4b) and also for 6 months (21.3 ± 1.7; Fig. S5).
The effect of light conditions and ABA-treatment on T. pieninicum proliferation rate of shoots during regrowth on MS medium with 1.11 μM BAP and 0.14 μM NAA in the 1st (a) and 2nd (b) subculture after 9 months storage at 4 °C. Statistically significant differences assessed using Kruskal–Wallis test at p ≤ 0.05: to the control plants in the same light conditions are denoted *(p < 0.05), **(p < 0.01), ***(p < 0.001); between light conditions are denoted #(p < 0.05), ##(p < 0.01), ###(p < 0.001); between ABA treatment in the same light conditions are denoted ◊(p < 0.05), ◊◊(p < 0.01), ◊◊◊(p < 0.001)
The last stage of T. pieninicum post-storage regeneration was rooting shoots on the MS medium. Regardless of the storage variant, the shoots were able to root (Fig. 5), except for the few ABA variants stored for 3 and 6 months (Fig. S6). The highest percentage of rooted shoots was obtained after storage for 9 months in the dark with 76 μM ABA inside synseed (Fig. 5a). ABA treatment did not reduce the number of roots developing from the shoots obtained after storage, regardless of the storage time (Figs. 5b, S7), but inhibited roots elongation, regardless of its exposure method and concentration (Figs. 5c, S8). However, ABA did not affect the ability of the regenerants to acclimatize. All plantlets transferred to ex vitro conditions were able to acclimatize. The morphological appearance of the regenerants treated even with highest ABA concentration did not differ to the control plants and plantlets were able to flower after transfer to the field conditions (Fig. 6).
The effect of light conditions and ABA-treatment on T. pieninicum rooting ability of shoots on MS medium after 9 months storage at 4 °C assessed by % of rooted shoots (a), number of roots per rooted shoots (b) and length of roots (c). Statistically significant differences assessed using Kruskal–Wallis test at p ≤ 0.05: to the control plants in the same light conditions are denoted *(p < 0.05), **(p < 0.01), ***(p < 0.001); between light conditions are denoted #(p < 0.05), ##(p < 0.01), ###(p < 0.001); between ABA treatment in the same light conditions are denoted ◊(p < 0.05), ◊◊(p < 0.01), ◊◊◊(p < 0.001)
Biochemical analyzes
After 4-weeks preculture on medium supplemented with ABA in the optimal growth conditions it was indicated that ABA significantly decreased chlorophyll content in the multiplicated microshoots (Fig. 7a). The usage of the highest ABA concentration (76 μM) significantly increased accumulation of H2O2 (Fig. 7b) but in none of the used concentration ABA affected soluble sugars accumulation (Fig. 7c). Treatment of the explants with ABA in the two highest concentrations (57 μM and 76 μM) significantly increased proline accumulation in the leaves (Fig. 7d).
Chlorophyll (a), H2O2 (b), soluble sugars (c) and proline (d) content in T. pieninicum shoots after 4-week preculture on medium MS supplemented with 1.11 μM BAP and 0.14 μM NAA and different concentration of ABA. Means with different letters are significantly different followed by Tukey test at p ≤ 0.05
After 9 months of storage the highest chlorophyll content was determined in control plants (Fig. 8a). The same results were obtained after 6 months of cooling, while after 3 months the highest chlorophyll accumulation was reported in leaves of shoots obtained from synseeds containing ABA in artificial endosperm (Fig. S9). Two-way ANOVA indicated that ABA application method had a significant effect on chlorophyll content after 3 and 6 months of storage, while ABA concentration had no significant effect regardless of the storage time (Table 1).
The effect of the light conditions and ABA-treatment on accumulation of chlorophyll (a), H2O2 (b), TBARS (c), soluble sugars (d) and proline (e) in T. pieninicum shoots directly after 9 months storage at 4 °C (a). Statistically significant differences assessed using Tukey test at p ≤ 0.05: to the control plants in the same light conditions are denoted *(p < 0.05), **(p < 0.01), ***(p < 0.001); between light conditions are denoted #(p < 0.05), ##(p < 0.01), ###(p < 0.001); between ABA treatment in the same light conditions are denoted ◊(p < 0.05), ◊◊(p < 0.01), ◊◊◊(p < 0.001)
Increasing ABA concentration in the preculture treatment led to an increase in the accumulation of the hydrogen peroxide in the plant tissue after 9 months of storage, what was particularly evident in the material stored under light conditions (Fig. 8b). A similar effect was noted for explants stored in capsules containing ABA under light conditions. In the dark ABA inside synseed had no effect on H2O2 levels. Furthermore, after storage in the dark in each ABA-treated variant there were no significant differences compared to the controls. A three-way ANOVA showed a significant individual effect after 9 months of storage of ABA treatment method, ABA concentration and light conditions on H2O2 accumulation (Table 2). However, the interaction of these factors did not significantly affect analyzed parameter. Preculture with ABA also increased H2O2 accumulation after 3 months storage (Fig. S10a). However statistically significant differences to the control was noted only for tissue stored in the dark. ABA addition inside synseed had no effect on H2O2 accumulation after 3 months storage. After 6 months of storage preculture with ABA and addition of ABA to the synseeds stored in the dark reduced H2O2 accumulation (Fig. S10b).
Directly after storage, TBARS content was also determined as a marker for lipid peroxidation. After 9 months of storage ABA inhibited TBARS accumulation in comparison to the control, especially when synseeds were stored under light conditions (Fig. 8c), although increasing ABA concentration significantly increased accumulation of determined compound. Three-way ANOVA showed not only significant effect of the light conditions on TBARS content, but also ABA concentration and method of its exposure (Table 3). After shorter storage time ABA did not indicated significant effect on TBARS content in plant tissue (Fig. S11).
The accumulation of soluble sugars depended on all analyzed variation factors (Fig. 8d; Table 4). After 9 months of storage under light conditions a significantly higher than in control plants level of soluble sugars was determined in explants treated with 38 μM ABA and 57 μM ABA during preculture. Increased sugar accumulation was also shown in leaves from variant where ABA was inside synseeds structure at a concentration of 19 μM and 38 μM and synseed were stored also under light conditions. After 6 months of storage ABA inhibited accumulation of soluble sugars in tissue cooled in both light conditions, while after 3 months ABA did not show any significant effect on analyzed parameter (Fig. S12).
The level of accumulated proline depended on the method of ABA treatment, its concentration and light conditions during storage (Fig. 8e; Table 5). After 9 months of storage proline level increased with an increase of ABA concentration inside synseeds structure, especially in light-stored explants. In contrast, the material treated with 19–57 μM ABA during preculture accumulated significantly less proline under light conditions than control plants. Similar results were obtained after 6 months of storage, but ABA did not affect proline accumulation after the shortest storage time (Fig. S13).
In response to the cold stress, endogenous ABA level increased significantly in shoots obtained from the synseeds non-treated with ABA stored under light conditions (38.0 ± 4.0 ng/g FW; Fig. 9a), while in the dark ABA accumulation after cooling (5.8 ± 2.6 ng/g FW) was comparable to the non-stored control (4.9 ± 0.6 ng/g FW; data not shown in the figure). The explants stored under light conditions and exposed to ABA at a concentration of 38 μM and 76 μM (both ABA-treatment) accumulated less endogenous ABA compared to the cold-stored control, while in the dark 38 μM ABA stimulated accumulation of endogenous ABA in the explants. Treatment with higher ABA concentration during storage in the dark did not affect the endogenous level of the phytohormone compared to the stored control and in addition determined level was comparable to non-stored control. Three-way ANOVA showed that the endogenous ABA level depended on ABA treatment method, its concentration and the light conditions (Table 6). A significant correlation between the examined factors was noted only for the ABA concentration and light conditions. Like ABA, the level of JA increased significantly in control shoots after cold-storage under the light conditions (187.4 ± 25.0 ng/g FW) in comparison to the non-stored control (31.2 ± 3.2 ng/g FW) (Fig. 9b). It was showed that ABA treatment, irrespective of its concentration and method of application, led to a statistically significant decrease in the level of JA in explants stored under light conditions. In shoots from all analyzed variants of synseed storage in the dark, the JA level was comparable to the control. Three-way ANOVA showed that light conditions had a significant effect on JA levels individually, as well as in correlation with ABA treatment method or ABA concentration (Table 6).
The effect of the light conditions and ABA treatment on the content of endogenous ABA (a) and JA (b) in shoots of T. pieninicum obtained from artificial seeds after storage for 9 months at 4 °C; the significance of differences was assessed by the Tukey test at α = 0.05 and determined: to the control plants in the same light conditions are denoted *(p < 0.05), **(p < 0.01), ***(p < 0.001); between light conditions are denoted #(p < 0.05), ##(p < 0.01), ###(p < 0.001); between ABA treatment in the same light conditions are denoted ◊(p < 0.05), ◊◊(p < 0.01), ◊◊◊(p < 0.001)
Discussion
Explant encapsulation used during storage of T. pieninicum is designed to protect plant tissue from low temperature damages and to physically reduce initial growth rate through an alginate coating (Kamińska et al. 2018a). Slowing the explants growth during storage is highly desirable due to the longer time without passage what reduce costs and the risk of contamination. The simplest method to slow down the growth of the plant material is to lower the culture temperature (Divakaran et al. 2006). Storage of synseeds at a reduced temperature was used to protect endangered species such as Celastrus paniculatus (Fonseka et al. 2019), Ceropegia barnesii (Ananthan et al. 2018), Cymbidium aloifolium (Pradhan et al. 2016), Eclipta alba (Salma et al. 2019), Mondia whitei (Baskaran et al. 2015), Spilanthes acmella (Sharma et al. 2009) and Withania coagulans (Rathore and Kheni 2017).
Morphological observations combined with biochemical analyzes have shown that light is a stress factor during storage of T. pieninicum synseeds, what was with agreement with our previous results also those obtained for non-encapsulated shoot tips of this species (Kamińska et al. 2016). Many studies indicated numerous relationships between light stress and low temperature conditions. Light-induced stress may result from an imbalance between the absorption and usage of a light energy (Ensminger et al. 2006; Beck et al. 2007). Absorption of a large amount of light in low metabolic activity conditions resulted from a lowered environmental temperature may led to damage of D1 protein, the site of attachment of plastoquinone QB. The inability to transfer energy to the PS reaction center may lead in turn to ROS production (Roach and Krieger-Liszkay 2014). Furthermore, under cold conditions electron transport may be inhibited by changing the structure of the cell membrane (Ensminger et al. 2006) and by reducing the activity of photosystem II (Borawska-Jarmułowicz et al. 2014) what may increase stress on which plant tissue is exposed.
Storage of synseeds in the dark seems to be a better method due to a more effective growth inhibition in comparison to the light conditions. This was confirmed for Hibiscus moscheutos synseeds which might be stored in the dark at 5 °C for almost 20 months with 80% viability (West et al. 2006) and for Splachnum ampullaceum synseeds stored under the same conditions for 30 months with maintaining viability of 50% explants (Mallón et al. 2007). Better results after storing the plant material in the dark compared to a light conditions were also obtained for Vitis berlandieri x V. riparia synthetic seeds after 9 months of cooling (Benelli 2016).
In order to further growth reduction of shoots during T. pieninicum synseeds storage in this study natural growth retardant abscisic acid was examined. Results showed that ABA limited shoots growth during the preculture preceding storage, as well as during synseeds storage (inside treatment) in both light variants. This is a potential indicator of the possibility of extending storage due to slower nutrient compounds consumption. Numerous studies indicated the inhibitory effect of ABA in plant in vitro culture. ABA inhibited the elongation growth of Citrus lemon (Kotsias and Roussos 2001), Oryza sativa (Pattanagul 2011) and Teucrium polium shoots (Rabba’a et al. 2012) during in vitro culture.
Numerous reports suggested that the addition of ABA to the medium allow extension of the storage time of viable explants e.g., Malus domestica (Kovalchuk et al. 2009), Tetrastigma hemsleyanum (Peng et al. 2015) or Lilium davidii and L. longiflorum (Yun-peng et al. 2012). However, medium supplementation with ABA reduced the survival of adventitious shoots of Vitis heyneana (Pan et al. 2014). Also in Physalis peruviana the addition of ABA to the synseeds reduced the survival of nodal cuttings and inhibited their elongation growth (Yücesan et al. 2015).
An important step in the in vitro preservation of plant material is the possibility of their micropropagation after storage. Regardless of the ABA treatment and duration of synseeds cold-storage, the shoot tips of T. pieninicum have been propagated under optimal growth conditions on the medium supplemented with BAP, but this process was more effective after storage in the dark, what was also noted after storage of non-encapsulated shoots of T. pieninicum (Kamińska et al. 2016). Similar results were published by Hausman et al. (1994) storing shoot tips of Populus tremula x P. tremuloides for 3 months at 4 °C in the dark without influence on shoot proliferation rate in comparison to non-stored control. Piccioni et al. (1996) reported regrowth of encapsulated explants of M.26 apple rootstock after 2 and 4 months at 4 °C. Effective propagation after synseeds storage was also noted for Olea europea and Quercus cerris after 6 weeks at 4 °C (Micheli et al. 1998; Tsvetkov and Hausman 2005), Corymbia torelliana × C. citriodora and Khana senegalensis after 12 months at 14˚C in the dark (Hung and Trueman 2012). ABA treatment did not affect T. pieninicum shoots ability to proliferate during post-storage regrowth. Similarly, addition of ABA to the medium did not affect the proliferation rate of Cedrus libani explants after 6 months of cold-storage (Renau-Morata et al. 2006). Also, the preculture of Castanea sativa shoots on the medium supplemented with 5 – 10 μM ABA did not affect their survival, but highest ABA concentration reduced shoots ability to proliferate after storage at 4 °C (Capuana and Di Leonardo 2013). In turn, in the study of da Silva and Scherwinski-Pereira (2011) the addition of ABA into MS medium reduced survival and proliferation rate of Piper aduncum and P. hispidinervum after 6 months of storage.
Taraxacum pieninicum shoots were effectively rooted on MS medium without auxin. Only shoots obtained after 3- and 6-months storage in capsule containing highest ABA concentration did not root. However, root organogenesis occurred after transfer of shoots to the vermiculite-sand mixture in ex vitro conditions what was also reported for other species from the Asteraceae family, e.g. Carlina acaulis (Trejgell et al. 2009).
Storage of the plant material using in vitro cultures, due to the presence of cold stresses and growth regulators (especially cytokinins) during regrowth stage may lead to genetic variability. However, during shoots multiplication from shoot tips usually the DNA level remained stable (Clarindo et al. 2008). In our previous research we verified the genetic stability of the plants obtained after long-term storage of non-encapsulated shoot tips on medium supplemented with ABA. No differences were observed using flow cytometry, RAPD, ISSR and SCoT markers between regrown plantlets after storage and plants cultivated from seeds in a soil (Kamińska et al. 2016, 2020).
The metabolic content changes under the influence of a low temperature may be associated with (a) catalytic activity or stability of enzymes involved in biosynthesis or degradation of specific compounds; (b) biosynthesis of compounds resulting from cellular damage; (c) regulating the concentration of given compounds to maintain homeostasis or (d) biosynthesis and accumulation of compounds involved in developing tolerance (Janmohammadi 2012). Therefore, the levels of chlorophyll, H2O2, TBARS / MDA, soluble sugars, proline as well as ABA and JA were analyzed.
Hordeum vulgare and Zea mays explants stored under high light intensity and low temperature were found to increase chlorophyll and xanthophyll content in response to ABA additions, what would indicated the role of ABA in protection of photosystem II (Jia and Lu 2003). The results presented in this paper did not indicate the participation of ABA in this process in T. pieninicum during storage in conditions of reduced light intensity because a decrease in Chl content in explants was noted. Furthermore, decreased level of Chl was also observed after preculture with ABA in optimal growth conditions without exposure to the low temperature. The cold conditions during storage of plant material also reduced the Chl content in Avena nuda seedlings (Liu et al. 2013). However, short-term storage of synseeds did not affect its level in T. pieninicum shoots obtained under optimal growth conditions. Similar results were reported for Rauvolfia tetraphylla synseeds stored for 4 weeks at reduced temperature (Faisal et al. 2013).
Numerous scientific reports indicated that under reduced temperature conditions there is an accumulation of ROS that leads to oxidative stress in plant tissues. Hydrogen peroxide is the most stable form of ROS, and its accumulation is considered as a direct response of plant cells to a low temperature (Carvajal et al. 2015). An increase in H2O2 content was noted after few hours of storage at 4 °C in Vitis vinifera (Rooy et al. 2017). In A. thaliana callus cells, the maximum increase in H2O2 accumulation was observed after 2 days at 4 °C, but from the 5th day of cooling, the level of H2O2 decreased (O’Kane et al. 1996). It was assumed that after the cessation of cold stress, the level of ROS decreases to the level occurring in optimal conditions (Einset et al. 2007). Excessive ROS accumulation can lead to chlorophyll degradation (Kavi Kishor and Sreenivasulu 2014), lipid peroxidation (Bhattacharjee 2005), and even to induction of programmed plant cell death (Desikan et al. 1998). Under stress conditions, level of endogenous ABA increase in plant tissue, what is one of the elements of the signal transductions pathway leading to an increase in chlorophyll photooxidation resistance (Pospíšilová et al. 2009) and activation of antioxidant enzymes (Jiang and Zhang 2001). Application of exogenous ABA may also reduce the effects of stress on plant tissue. However, in the case of T. pieninicum no such effect was found. A significant increase in H2O2 accumulation has been shown in the light, while ABA may not be involved in the production or neutralization of ROS in this species. However, high concentration of ABA increased H2O2 level in T. pieninicum leaves grown under optimal growth conditions.
The consequence of ROS accumulation may be a peroxidation of membrane lipids which is denoted by the accumulation of malonodialdehyde (Liu et al. 2013; Rooy et al. 2017). T. pieninicum explants stored in the light accumulated significantly more TBARS / MDA than in the dark. In addition, its level in plant tissue increased with prolonging storage time. The ABA application did not affect the level of the tested parameter after 3 and 6 months of storage. However, after 9 months significantly less TBARS/MDA was determined in plant tissue treated with ABA than in the control, especially after storage under light conditions, what may indicated the involvement of ABA in lipid peroxidation inhibition mechanisms. Similar results were obtained for Saccharum officinarum (Huang et al. 2015) and Vitis vinifera (Karimia et al. 2016), where foliar application of 50–200 μM ABA led to a decrease in TBARS content under reduced temperature conditions.
An increased content of soluble sugars during acclimation to cold has been observed for many species, including A. thaliana (Klotke et al. 2004), Triticum aestivum (Kamata and Uemura 2004), Hordeum vulgare (Tabaei-Aghdaei et al. 2003) and Solanum tuberosum (Folgado et al. 2013). Tognetti et al. (1990) proposed that the increased accumulation of sugars in plant tissue enhanced resistance of the species to a low temperature. After 3 months of storage of T. pieninicum no effect of ABA on the accumulation of soluble sugars in tissue was noted. Additionally, treatment of explants with ABA during preculture and prolonged storage for up to 9 months in the dark also did not affect the level of accumulated sugars compared to controls. In contrast, literature reports point to a relationship between ABA accumulation and soluble sugars under cold stress (Rooy et al. 2017).
Accumulation of proline in the tissues of cooled plant material has proven to be of key importance in the process of cold acclimation, what has been shown for many species, including Saccharum officinarum (Rasheed et al. 2010), Vitis vinifera (Rooy et al. 2017) and Avena nuda (Liu et al. 2013). However, the increased proline accumulation does not necessarily indicate a high tolerance to coldness or freezing of a given species, but it is necessary to adapt the tissue to a condition that may lead to a disruption of its growth (Rooy et al. 2017). In Quercus rober proline abolished the inhibitory effect of cold on the growth of embryogenic tissue (Gleeson et al. 2004), while in Cicer arietinum proline stimulated the accumulation of sucrose and chlorophyll in plants exposed to low temperature (Kaur et al. 2011). Accumulation of proline did not depend on storage time in T. pieninicum. Higher proline content was determined in light-stored tissue than in the dark, what confirmed that light combine with the low temperature causes severe stress in this species. Treatment of ABA explants did not significantly affect proline accumulation during storage in the dark. However, the stimulating effect of ABA on proline accumulation was determined in shoots obtained from synseeds containing 76 μM ABA in artificial endosperm and stored for 9 months under light conditions. Also, high ABA concentration resulted in increased proline accumulation after preculture under optimal growth conditions. Numerous studies indicated that ABA led to increased proline accumulation (Kavi Kishor and Sreenivasulu 2014), such a relationship was noted for example for Triticum aestivum (Hou et al. 2010).
Exposure of T. pieninicum tissue to the low temperature led to a significant increase in endogenous ABA and JA only after storage under light conditions. It is well-known that the level of endogenous ABA in plant material can be controlled by light (Xu et al. 2014; Seo et al. 2009). In our study reduced shoot proliferation after storage under light conditions may probably resulted from the high levels of endogenous ABA in tissue. However, no significant differences between light conditions during storage and ABA accumulation were noted. It seems that the response of this species to cold-storage under continuous light is more complex and does not depend only on the accumulation of ABA in tissue. Treatment of explants with ABA did not lead to an increase in endogenous ABA accumulation in tissue compared to the cooled control. Under low temperature conditions, an increase in endogenous ABA levels was noted in Oryza sativa (Oliver et al. 2007) and Vitis vinifera (Rooy et al. 2017). In Actinidia deliciosa endogenous ABA levels increased until the second month of cooling at 0 ± 0.5 °C. However, longer storage led to a decrease in ABA accumulated levels (Yang et al. 2013). In Arabidopsis exposure to JA led to a decrease in ABA accumulation, while exogenous ABA under abiotic stress stimulated JA biosynthesis (Adie et al. 2007; Fan et al. 2009; Wang et al. 2018). However, treatment of T. pieninicum tissue with ABA led to significantly lower JA content after light storage.
Summing up the results, it can be stated that treatment with ABA reduces the effects of cold stress in T. pieninicum tissues stored in the light, what was manifested by reduced H2O2 levels in shoots subjected to the preculture on medium supplemented with ABA (19–57 μM). In addition, significant reduction of membrane lipid peroxidation was noted after tissue treatment with ABA before and during cold-storage. No stimulating effect of treatment with ABA on the level of chlorophyll and the proliferation rate of cooled shoots was observed. Signal transduction pathways of ABA and JA are interrelated because treatment of ABA tissue significantly reduced JA content. ABA and JA are involved in the protective mechanisms of plant tissues against low temperature stress, however, processes associated with acclimatization to the cold are multifactorial, and the studied phytohormones are not the only factors affecting the expression of genes associated with the plant's response to cold. Potential participation in this process might be also attributed to ethylene (Zhao et al. 2014), gibberellic acid (Richter et al. 2013), brassinosteroids (Singh et al. 2012), salicylic acid (Dong et al. 2014), ROS and cytosolic Ca2+ (Chinnusamy et al. 2007).
Conclusions
In conclusion, light combined with low temperature is a stress factor during storage of T. pieninicum, regardless of the ABA exposure. Treatment with abscisic acid of T. pieninicum explants limits their growth, which contributes to the extension of their storage time at reduced temperature at least to 9 months and alleviates the effects of cold stress. Shoots of T. pieninicum after cold storage and ABA treatment are able to regrowth effectively under optimal growth conditions, while field acclimatized regenerants after storage and propagation in vitro are able to flower.
Abbreviations
- ABA:
-
Abscisic acid
- BAP:
-
Benzylaminopurine
- Chl :
-
Chlorophyll
- JA:
-
Jasmonic acid
- MDA:
-
Malondialdehyde
- NAA:
-
Naphthalene acetic acid
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
References
Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681. https://doi.org/10.1105/tpc.106.048041
Ananthan R, Mohanraj R, Bai VN (2018) In vitro regeneration, production, and storage of artificial seeds in Ceropegia barnesii, an endangered plant. Vitro Cell Dev Biol - Plant 54:553–563. https://doi.org/10.1007/s11627-018-9934-x
Baskaran P, Kumari A, van Staden J (2015) Embryogenesis and synthetic seed production in Mondia whitei. Plant Cell Tiss Organ Cult 121:205–214. https://doi.org/10.1007/s11240-014-0695-x
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32:501–510. https://doi.org/10.1007/s12038-007-0049-5
Benelli C (2016) Encapsulation of shoot tips and nodal segments for in vitro storage of “Kober 5BB” grapevine rootstock. Horticulture 2:10. https://doi.org/10.3390/horticulturae2030010
Bhattacharjee S (2005) Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Curr Sci 89:1113–1121
Borawska-Jarmułowicz B, Mastalerczuk G, Pietkiewicz S, Kalaji MH (2014) Low temperature and hardening effects on photosynthetic apparatus efficiency and survival of forage grass varieties. Plant Soil Environ 60:177–183. https://doi.org/10.17221/57/2014-PSE
Capuana M, Di Leonardo S (2013) In vitro conservation of chestnut (Castanea sativa) by slow growth. Vitro Cell Dev Biol - Plant 49:605–610. https://doi.org/10.1007/s11627-013-9536-6
Carvajal F, Palma F, Jamilena M, Garrido D (2015) Preconditioning treatment induces chilling tolerance in zucchini fruit improving different physiological mechanisms against cold injury. Ann Appl Biol 166:340–354. https://doi.org/10.1111/aab.12189
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451. https://doi.org/10.1016/j.tplants.2007.07.002
Clarindo WR, Carvalho CR, Araujo FS, Abreu IS, Otoni WC (2008) Recovering polyploid papaya in vitro regenerants as screened by flow cytometry. Plant Cell Tiss Organ Cult 92:207–214. https://doi.org/10.1007/s11240-007-9325-1
Cruz-Cruz CA, González-Arnao MT, Engelmann F (2013) Biotechnology and conservation of plant biodiversity. Resources 2:73–95. https://doi.org/10.3390/resources2020073
da Silva TL, Scherwinski-Pereira JE (2011) In vitro conservation of Piper aduncum and Piper hispidinervum under slow-growth conditions. Pesq Agropec Bras 46:384–389. https://doi.org/10.1590/S0100-204X2011000400007
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330:115–120. https://doi.org/10.1042/bj3300115
Divakaran M, Babu KN, Peter KV (2006) Conservation of Vanilla species, in vitro. Sci Hortic 110:175–180. https://doi.org/10.1016/j.scienta.2006.07.003
Dong CJ, Li L, Shang QM, Liu XY, Zhang ZG (2014) Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 240:687–700. https://doi.org/10.1007/s00425-014-2115-1
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Einset JNE, Connolly EL, Bones A, Sparstad T, Winge P, Zhu JK (2007) Membrane-trafficking RabA4c involved in the effect of glycine betaine on recovery from chilling stress in Arabidopsis. Physiol Plant 130:511–518. https://doi.org/10.1111/j.1399-3054.2007.00920.x
Ensminger I, Busch F, Huner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126:28–44. https://doi.org/10.1111/j.1399-3054.2006.00627.x
Espindula MC, Rocha VS, Grossi JAS, Souza MA, Souza LT, Favarato LF (2009) Use of growth retardants in wheat. Planta Daninha 27:379–387. https://doi.org/10.1590/S0100-83582009000200022
Faisal M, Awatar AA, Pegazy AK (2013) Molecular and biochemical characterization in Rauvolfia tetraphylla plantlets grown from synthetic seeds following in vitro cold storage. Appl Biochem Biotechnol 169:408–417. https://doi.org/10.1007/s12010-012-9977-0
Fan J, Hill L, Crooks C, Doerner P, Lamb C (2009) Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 150:1750–1761. https://doi.org/10.1104/pp.109.137943
Folgado R, Panis B, Hausman J (2013) Differential protein expression in response to abiotic stress in two potato species: Solanum commersonii Dun and Solanum tuberosum L. Int J Mol Sci 14:4912–4933. https://doi.org/10.3390/ijms14034912
Fonseka DLCK, Wickramaarachchi WWUI, Madushani RPS (2019) Synthetic seed production as a tool for the conservation and domestication of Celastrus paniculatus: a rare medicinal plant. Annu Res Rev Biol 32:1–8. https://doi.org/10.9734/arrb/2019/v32i430092
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525. https://doi.org/10.1007/s10265-011-0412-3
Gleeson D, Lelu-Walter M, Parkinson M (2004) Influence of exogenous L-proline on embryogenic cultures of larch (Larix leptoeuropaea Dengler), sitka spruce (Picea sitchensis (Bong.) Carr.) and oak (Quercus robur L.) subjected to cold and salt stress. Ann For Sci 61:125–128. https://doi.org/10.1051/forest:2004003
Hausman JF, Neys O, Kevers C, Gaspar T (1994) Effect of in vitro storage at 4˚C on survival and proliferation of poplar shoots. Plant Cell Tiss Organ Cult 38:65–67. https://doi.org/10.1007/BF00034446
Heinrich M, Hettenhausen C, Lange T, Wünsche H, Fang J, Baldwin IT, Wu J (2012) High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuate stems. Plant J 73:591–606. https://doi.org/10.1111/tpj.12058
Honek A, Martinkova Z, Saska P (2005) Post-dispersal predation of Taraxacum officinale (dandelion) seed. J Ecol 93:345–352. https://doi.org/10.1111/j.1365-2745.2005.00987.x
Hou YD, Guo ZF, Yi Y, Li HN, Li HG, Chen LJ, Ma H, Zhang L, Lin JW, Zhong M (2010) Effects of cold acclimation and exogenous phytohormones abscisic acid treatment on physiological indicators of winterness wheat. J Plant Sci 5:125–136. https://doi.org/10.3923/jps.2010.125.136
Huang X, Chen MH, Yang LT, Li YR, Wu JM (2015) Effects of exogenous abscisic acid on cell membrane and endogenous hormone contents in leaves of sugarcane seedling under cold stress. Sugar Tech 17:59–64. https://doi.org/10.1007/s12355-014-0343-0
Humplík JF, Bergougnoux V, van Volkenburgh E (2017) To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci 22:830–841. https://doi.org/10.1016/j.tplants.2017.07.009
Hung CD, Trueman SJ (2012) Alginate encapsulation of shoot tips and nodal segments for short-term storage and distribution of the eucalypt Corymbia torelliana x C. citriodora. Acta Physiol Plant 34:117–128. https://doi.org/10.1007/s11738-011-0810-0
Janmohammadi M (2012) Metabolomic analysis of low temperature responses in plants. Curr Opin Agric 1:1–6
Jia H, Lu C (2003) Effects of abscisic acid on photoinhibition in maize plants. Plant Sci 165:1403–1410. https://doi.org/10.1016/j.plantsci.2003.08.004
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273. https://doi.org/10.1093/pcp/pce162
Kamata T, Uemura M (2004) Solute accumulation in heat seedlings during cold acclimation: contribution to increased freezing tolerance. Cryo Lett 25:311–322
Kamińska M, Skrzypek E, Wilmowicz E, Tretyn A, Trejgell A (2016) Effect of light conditions and ABA on cold storage and post-storage propagation of Taraxacum pieninicum. Plant Cell Tiss Organ Cult 127:25–34. https://doi.org/10.1007/s11240-016-1026-1
Kamińska M, Gołębiewski M, Tretyn A, Trejgell A (2018a) Efficient long-term conservation of Taraxacum pieninicum synthetic seeds in slow growth conditions. Plant Cell Tiss Organ Cult 132:469–478. https://doi.org/10.1007/s11240-017-1343-z
Kamińska M, Tretyn A, Trejgell A (2018b) Effect of jasmonic acid on cold-storage of Taraxacum pieninicum encapsulated shoot tips. Plant Cell Tiss Organ Cult 135:487–497. https://doi.org/10.1007/s11240-018-1481-y
Kamińska M, Tretyn A, Trejgell A (2020) Genetic stability assessment of Taraxacum pieninicum plantlets after long-term slow growth storage using ISSR and SCoT markers. Biologia 75:599–604. https://doi.org/10.2478/s11756-019-00377-x
Karimia R, Ershadi A, Nejadb AR, Khanizadeh S (2016) Abscisic acid alleviates the deleterious effects of cold stress on ‘Sultana’ grapevine (Vitis vinifera L.) plants by improving the antioxidant activity and photosynthetic capacity of leaves. J Hortic Sci Biotechnol 91:386–395. https://doi.org/10.1080/14620316.2016.1162027
Kaur G, Kumar S, Thakur P, Malik JA, Bhandhari K, Sharma KD, Nayyar H (2011) Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci Hortic 128:174–181. https://doi.org/10.1016/j.scienta.2011.01.037
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37:300–311. https://doi.org/10.1111/pce.12157
Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61:561–591. https://doi.org/10.1146/annurev-arplant-042809-112226
Klotke J, Kopka J, Gatzke N, Heyer AG (2004) Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation evidence for a role of raffinose in cold acclimation. Plant Cell Environ 27:1395–1404. https://doi.org/10.1111/j.1365-3040.2004.01242.x
Kotsias D, Roussos PA (2001) An investigation on the effect of different plant growth regulating compounds in in vitro shoot tip and node culture of lemon seedlings. Sci Hortic 89:115–128. https://doi.org/10.1016/S0304-4238(00)00227-2
Kovalchuk I, Lyudvikova Y, Volgina M, Reed BM (2009) Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell Tiss Organ Cult 96:127–136. https://doi.org/10.1007/s11240-008-9468-8
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Liu W, Yu K, He T, Li F, Zhang D, Liu J (2013) The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci World J 2013:658793. https://doi.org/10.1155/2013/658793
Lorrai R, Boccaccini A, Ruta V, Possenti M, Costantino P, Vittorioso P (2018) Abscisic acid inhibits hypocotyl elongation acting on gibberellins DELLA proteins and auxin. AoB Plants 10:ply061. https://doi.org/10.1093/aobpla/ply061
Mallón R, Barros P, Luzardo A, González ML (2007) Encapsulation of moss buds: an efficient method for the in vitro conservation and regeneration of the endangered moss Splachnum ampullaceum. Plant Cell Tiss Organ Cult 88:41–49. https://doi.org/10.1007/s11240-006-9176-1
Micheli M, Mencuccini M, Standardi A (1998) Encapsulation of in vitro proliferated buds of olive. Adv Hortic Sci 12:163–168
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:437–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nair PMG, Chung IM (2015) Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant 37:1719. https://doi.org/10.1007/s11738-014-1719-1
O’Kane D, Gill V, Boyd P, Burdon R (1996) Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta 198:371–377. https://doi.org/10.1007/BF00620053
Oliver SN, Dennis ES, Dolferus R (2007) ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol 48:1319–1330. https://doi.org/10.1093/pcp/pcm100
Palva ET, Thtiharju S, Tamminen I, Puhakainen T, Laitinen R, Svensson J, Helenius E, Heino P (2002) Biological mechanisms of low temperature stress response: cold acclimation and development of freezing tolerance in plants. JIRCAS Working Report, pp 9–15
Pan XJ, Zhang WE, Li X (2014) In vitro conservation of native Chinese wild grape (Vitis heyneana Roem. & Schult) by slow growth culture. Vitis 53:207–214
Pattanagul W (2011) Exogenous abscisic acid enhance sugar accumulation in rice (Oryza sativa L.) under drought stress. Asian J Plant Sci 10:212–219. https://doi.org/10.3923/ajps.2011.212.219
Pence VC (2010) Evaluating costs for in vitro propagation and preservation of endangered plants. In Vitro Cell Dev Biol - Plant 47:176–187. https://doi.org/10.1007/s11627-010-9323-6
Peng X, Ji Q, Wu H, Li Y (2015) Slow-growth conservation and clonal fidelity of Tetrastigma hemsleyanum microplants. In Vitro Cell Dev Biol - Plant 51:463–470. https://doi.org/10.1007/s11627-015-9709-6
Piccioni E, Standardi A, Tutuianu VC (1996) Storage of M.26 apple rootstock encapsulated microcuttings. Adv Hortic Sci 10:185–190
Piękoś-Mirkowa H, Mirek Z (2009) Distribution patterns and habitats of endemic vascular plants in the Polish Carpathians. Acta Soc Bot Poloniae 78:321–326. https://doi.org/10.5586/asbp.2009.042
Pospíšilová J, Haisel D, Synková H, Baťková-Spoustová P (2009) Improvement of ex vitro transfer of tobacco plantlets by addition of abscisic acid to the last subculture. Biol Plant 54:617–624. https://doi.org/10.1007/s10535-009-0113-0
Pradhan S, Tiruwa BL, Subedee BR, Pant B (2016) Efficient plant regeneration of Cymbidium aloifolium (L.) Sw., a threatened orchid of Nepal through artificial seed technology. Am J Plant Sci 7:1964–1974. https://doi.org/10.4236/ajps.2016.714179
Puchalski J, Nowak A, Smieja A, Rucińska A, Kapler A, Niemczyk M, Walerowski P, Krzyżewski A (2014) Flagship species of the Pieniny NP. protected ex situ at PAS BG CBDC seed bank at Warsaw-Powsin. Nat J 47:9–21
Rabba’a MM, Shibli RA, Shatnawi MA (2012) In vitro medium term conservation of felty germander (Teucrium polium L.) micro-shoots. Jordan J Agric Sci 8:523–534
Rasheed R, Wahid A, Ashraf M, Basra SMA (2010) Role of proline and glycinebetaine in improving chilling stress tolerance in sugarcane buds at sprouting. Int J Agric Biol 12:1–8
Rathore MS, Kheni J (2017) Alginate encapsulation and in vitro plantlet regeneration in critically endangered medicinal plant, Withania coagulans (Stocks) Dunal. J Proc Natl Acad Sci India Sect B 87:129–134. https://doi.org/10.1007/s40011-015-0577-y
Renau-Morata B, Arrillaga I, Segura J (2006) In vitro storage of cedar shoot cultures under minimal growth conditions. Plant Cell Rep 25:636–642. https://doi.org/10.1007/s00299-006-0129-2
Richter R, Bastakis E, Schwechheimer C (2013) Cross-repressive interactions between SOC1 and the GATAs GNC and GNL/CGA1 in the control of greening, cold tolerance, and flowering time in Arabidopsis. Plant Physiol 162:1992–2004. https://doi.org/10.1104/pp.113.219238
Roach T, Krieger-Liszkay A (2014) Regulation of photosynthetic electron transport and photoinhibition. Curr Protein Pept Sci 15:351–362. https://doi.org/10.2174/1389203715666140327105143
Rooy SSB, Salekdeh GH, Ghabooli M, Gholami M, Karimi R (2017) Cold-induced physiological and biochemical responses of three grapevine cultivars differing in cold tolerance. Acta Physiol Plant 39:264. https://doi.org/10.1007/s11738-017-2561-z
Sah SK, Reddy KR, Li J (2016) Abscisic acid and Abiotic stress tolerance in crop plants. Front Plant Sci 7:571. https://doi.org/10.3389/fpls.2016.00571
Salma U, Kundu S, Ali MN, Mandal N (2019) Somatic embryogenesis-mediated plant regeneration of Eclipta alba (L.) Hassk and its conservation through synthetic seed technology. Acta Physiol Plant 41:103. https://doi.org/10.1007/s11738-019-2898-6
Schütz K, Carle R, Schieber A (2006) Taraxacum—a review on its phytochemical and pharmacological profile. J Ethnopharmacol 107:313–323. https://doi.org/10.1016/j.jep.2006.07.021
Seo M, Nambara E, Choi G, Yamaguchi S (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69:463–472. https://doi.org/10.1007/s11103-008-9429-y
Sharma S, Shahzad A, Sahai A (2009) Artificial seeds for propagation and preservation of Spilanthes acmella (L.) Murr., a threatened pesticidal plant species. Int J Plant Dev Biol 3:62–64
Singh I, Kumar U, Singh SK, Gupta C, Singh M, Kushwaha SR (2012) Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol Mol Biol Plants 18:229–236. https://doi.org/10.1007/s12298-012-0122-x
Song SY, Chen Y, Chen J, Dai XY, Zhang WH (2011) Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234:331–345. https://doi.org/10.1007/s00425-011-1403-2
Tabaei-Aghdaei SR, Pearce RS, Harrison P (2003) Sugars regulate cold-induced gene expression and freezing-tolerance in barley cell cultures. J Exp Bot 54:1565–1575. https://doi.org/10.1093/jxb/erg173
Tognetti JA, Salerno GL, Crespi MD, Pontis HG (1990) Sucrose and fructan metabolizm of different wheat cultivars at chilling temperatures. Physiol Plant 78:554–559. https://doi.org/10.1111/j.1399-3054.1990.tb05241.x
Trejgell A, Bednarek M, Tretyn A (2009) Micropropagation of Carlina acaulis L. Acta Biol Cracov Bot 51:97–103
Trejgell A, Chernetskyy M, Podlasiak J, Tretyn A (2013) An efficient system for regenerating Taraxacum pieninicum Pawł. from seedling explants. Acta Biol Cracov Ser Bot 55:73–79. https://doi.org/10.2478/abcsb-2013-00013
Tsvetkov I, Hausman JF (2005) In vitro regeneration from alginate-encapsulated microcuttings of Quercus sp. Sci Hortic 103:503–507. https://doi.org/10.1016/j.scienta.2004.06.013
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wang K, Guo Q, Froehlich JE, Hersh HL, Zienkiewicz A, Howe GA, Benning C (2018) Two abscisic acid-responsive plastid lipase genes involved in jasmonic acid biosynthesis in Arabidopsis thaliana. Plant Cell 30:1006–1022. https://doi.org/10.1105/tpc.18.00250
West TP, Ravindra MB, Preece JE (2006) Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell Tiss Organ Cult 87:223–231. https://doi.org/10.1007/s11240-006-9155-6
Xu D, Li J, Gangappa SN, Hettiarachchi C, Lin F, Andersson MX, Jiang Y, Deng XW, Holm M (2014) Convergence of light and ABA signaling on the ABI5 promoter. PLoS Genet 10:e1004197. https://doi.org/10.1371/journal.pgen.1004197
Yang Q, Zhang Z, Rao J, Wang Y, Sun Z, Ma Q, Dong X (2013) Low-temperature conditioning induces chilling tolerance in ‘Hayward’ kiwifruit by enhancing antioxidant enzyme activity and regulating endogenous hormones levels. J Sci Food Agric 93:3691–3699. https://doi.org/10.1002/jsfa.6195
Yücesan BB, Mohammed A, Arslan M, Ekrem G (2015) Clonal propagation and synthetic seed production from nodal segments of Cape gooseberry (Physalis peruviana L.), a tropical fruit plant. Turk J Agric For 39:797–806. https://doi.org/10.3906/tar-1412-86
Yun-peng D, Wen-yuan L, Ming-fang Z, Heng-bin H, Gui-xia J (2012) The establishment of a slow-growth conservation system in vitro for two wild lily species. Afr J Biotechnol 11:1981–1990. https://doi.org/10.5897/AJB11.2868
Zhao M, Liu W, Xia X, Wang T, Zhang WH (2014) Cold acclimation-induced freezing tolerance of Medicago truncatula seedling is negatively regulated by ethylene. Physiol Plant 152:115–129. https://doi.org/10.1111/ppl.12161
Acknowledgements
This project was supported by fund from Ministry of Science and Higher Education (PL) for Nicolaus Copernicus University in Toruń, including a Grant for young scientist (MK) number 2827-B.
Author information
Authors and Affiliations
Contributions
MK designed and carried out all the experiments, analyzed the data and wrote the manuscript, JK helped in analysis of endogenous phytohormones level and AT helped in analysis of the data and in writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Maurizio Lambardi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamińska, M., Kęsy, J. & Trejgell, A. Abscisic acid in preservation of Taraxacum pieninicum in the form of synthetic seeds in slow growth conditions. Plant Cell Tiss Organ Cult 144, 295–312 (2021). https://doi.org/10.1007/s11240-020-01924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01924-0