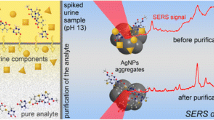
Abstract
This report is dedicated to determination of anticancer drug methotrexate (MTX) in human urine using surface-enhanced Raman spectroscopy (SERS). Aluminum oxide loaded with silver nanoparticles (AO-Ag) was proposed as SERS-active sorbent and used for solid-phase extraction (SPE) of the analyte and its SERS-based determination (SPE-SERS protocol). MTX has strong SERS signal only in alkaline media that challenges its determination in urine due to strong background signal caused by creatinine. The application of SPE step enables to purify and concentrate the analyte making MTX determination possible. Also, the application of the same material for SPE pretreatment and SERS analysis enables to simplify and speed-up the protocol. The protocol was developed and tested using artificially spiked samples of human urine collected during different time of day to account deviating composition of the urine matrix. The use of dilution step of the analyte-containing urine was proposed prior SPE-SERS protocol to reduce the difference between morning-time- and daytime-collected urine achieving maximal reliability of the analysis. Additional physicochemical study was performed to estimate an influence of the primary intrinsic urine components (salts, urea, creatinine) and their mixtures on the analytical signal. Final protocol enables MTX determination in human urine within 20–300 μg mL−1 range of concentrations with satisfactory precision (11–19% RSD), accuracy (97–104% apparent recovery), and limit of detection (4.2 μg mL−1). Accounting that the analysis requires less than 15 min and portable Raman spectrometer, the protocol seems to be promising for therapeutic drug monitoring in hospitals to identify poor MTX clearance in a timely manner and minimize adverse effects of therapy.

Graphical Abstract






Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bonifacio A, Cervo S, Sergo V. Label-free surface-enhanced Raman spectroscopy of biofluids: fundamental aspects and diagnostic applications. Anal Bioanal Chem. 2015;407:8265–77.
Zheng XS, Jahn IJ, Weber K, Cialla-May D, Popp J. Label-free SERS in biological and biomedical applications: recent progress, current challenges and opportunities. Spectrochim Acta A. 2018;197:56–77.
Jaworska A, Fornasaro S, Sergo V, Bonifacio A. Potential of surface enhanced Raman spectroscopy (SERS) in therapeutic drug monitoring (TDM). A critical review. Biosensors. 2016;6:47.
Frosch T, Knebl A, Frosch T. Recent advances in nano-photonic techniques for pharmaceutical drug monitoring with emphasis on Raman spectroscopy. Nanophotonics. 2020;9:19–37.
Li W, Zhao X, Yi Z, Glushenkov AM, Kong L. Plasmonic substrates for surface enhanced Raman scattering. Anal Chim Acta. 2017;984:19–41.
Markin AV, Markina NE, Popp J, Cialla-May D. Copper nanostructures for chemical analysis using surface-enhanced Raman spectroscopy. Trends Anal Chem. 2018;108:247–59.
Yang L, Li P, Liu J. Progress in multifunctional surface-enhanced Raman scattering substrate for detection. RSC Adv. 2014;4:49635–46.
Chen M, Luo W, Zhang Z, Wang R, Zhu Y, Yang H, et al. Synthesis of multi-Au-nanoparticle-embedded mesoporous silica microspheres as self-filtering and reusable substrates for SERS detection. ACS Appl Mater Interfaces. 2017;9:42156–66.
Zhang Q, Li D, Cao X, Gu H, Deng W. Self-assembled microgels arrays for electrostatic concentration and surface-enhanced Raman spectroscopy detection of charged pesticides in seawater. Anal Chem. 2019;91:11192–9.
Alvarez-Puebla RA, Liz-Marzán LM. Traps and cages for universal SERS detection. Chem Soc Rev. 2012;41:43–51.
Innocenzi P, Malfatti L. Mesoporous materials as platforms for surface-enhanced Raman scattering. Trends Anal Chem. 2019;114:233–41.
Guo X, Li J, Arabi M, Wang X, Wang Y, Chen L. Molecular-imprinting-based surface-enhanced Raman scattering sensors. ACS Sens. 2020;5:601–19.
Markina NE, Volkova EK, Zakharevich AM, Goryacheva IY, Markin AV. SERS detection of ceftriaxone and sulfadimethoxine using copper nanoparticles temporally protected by porous calcium carbonate. Microchim Acta. 2018;185:481.
Kang H, Buchman JT, Rodriguez RS, Ring HL, He J, Bantz KC, et al. Stabilization of silver and gold nanoparticles: preservation and improvement of plasmonic functionalities. Chem Rev. 2019;119:664–99.
Contreras-Cáceres R, Abalde-Cela S, Guardia-Girós P, Fernández-Barbero A, Pérez-Juste J, Alvarez-Puebla RA, et al. Multifunctional microgel magnetic/optical traps for SERS ultradetection. Langmuir. 2011;27:4520–5.
Markina NE, Markin AV, Zakharevich AM, Goryacheva IY. Calcium carbonate microparticles with embedded silver and magnetite nanoparticles as new SERS-active sorbent for solid phase extraction. Microchim Acta. 2017;184:3937–44.
Aldeanueva-Potel P, Faoucher E, Alvarez-Puebla RA, Liz-Marzan LM, Brust M. Recyclable molecular trapping and SERS detection in silver-loaded agarose gels with dynamic hot spots. Anal Chem. 2009;81:9233–8.
Qu LL, Wang N, Zhu G, Yadav TP, Shuai X, Bao D, et al. Facile fabrication of ternary TiO2-gold nanoparticle-graphene oxide nanocomposites for recyclable surface enhanced Raman scattering. Talanta. 2018;186:265–71.
Yue S, Sun XT, Wang Y, Zhang WS, Xu ZR. Microparticles with size/charge selectivity and pH response for SERS monitoring of 6-thioguanine in blood serum. Sensors Actuators B Chem. 2018;273:1539–47.
Yurova NS, Markina NE, Pozharov MV, Zakharevich AM, Rusanova TY, Markin AV. SERS-active sorbent based on aluminum oxide loaded with silver nanoparticles. Colloids Surf A Physicochem Eng Asp. 2016;495:169–75.
Leopold N, Lendl B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J Phys Chem B. 2003;107:5723–7.
Hidi IJ, Jahn M, Pletz MW, Weber K, Cialla-May D, Popp J. Toward levofloxacin monitoring in human urine samples by employing the LoC-SERS technique. J Phys Chem C. 2016;120:20613–23.
Bratlid D, Moe PJ. Pharmacokinetics of high-dose methotrexate treatment in children. Eur J Clin Pharmacol. 1978;14:143–7.
Hidi IJ, Mühlig A, Jahn M, Liebold F, Cialla D, Weber K, et al. LOC-SERS: towards point-of-care diagnostic of methotrexate. Anal Methods. 2014;6:3943–7.
Markina NE, Markin AV, Weber K, Popp J, Cialla-May D. Liquid-liquid extraction-assisted SERS determination of sulfamethoxazole in spiked human urine. Anal Chim Acta. 2020;1109:61–8.
Breithaupt H, Kuenzelen E. Pharmacokinetics of methotrexate and 7-hydroxymethotrexate following infusions of high-dose methotrexate. Cancer Treat Rep. 1982;66:1733–41.
Seideman P, Beck O, Eksborg S, Wennberg M. The pharmacokinetics of methotrexate and its 7-hydroxy metabolite in patients with rheumatoid arthritis. Br J Clin Pharmacol. 1993;35:409–12.
Zhang R, Gellman AJ. Straight-chain alcohol adsorption of the silver(110) surface. J Phys Chem. 1991;95:7433–7.
Subaihi A, Trivedi DK, Hollywood KA, Bluett J, Xu Y, Muhamadali H, et al. Quantitative online liquid chromatography−surface-enhanced Raman scattering (LC-SERS) of methotrexate and its major metabolites. Anal Chem. 2017;89:6702–9.
Fornasaro S, Dalla Marta S, Rabusin M, Bonifacio A, Sergo V. Toward SERS-based point-of-care approaches for therapeutic drug monitoring: the case of methotrexate. Faraday Discuss. 2016;187:485–99.
Marsich L, Bonifacio A, Mandal S, Krol S, Beleites C, Sergo V. Poly-l-lysine-coated silver nanoparticles as positively charged substrates for surface-enhanced Raman scattering. Langmuir. 2012;28:13166–71.
Markina NE, Markin AV. Application of aluminum hydroxide for improvement of label-free SERS detection of some cephalosporin antibiotics in urine. Biosensors. 2019;9:91.
Funding
The work was supported by Russian Science Foundation (project 18-13-00081).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Samples of human urine were provided by healthy volunteers (4 males and 2 females, 25–40 age range) and collected as spontaneously voided portions. All volunteers were provided with the complete description of the study and signed informed consent was collected from each volunteer before providing the urine samples. The samples were anonymized retaining only information about time of collection (morning-time- or daytime-collected urine). Approving the study by ethics committee was not performed because the analyte (methotrexate—an anticancer drug) was used only for artificial (in vitro) spiking of the collected urine samples and the participants did not administer it.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1853 kb).
Rights and permissions
About this article
Cite this article
Markina, N.E., Zakharevich, A.M. & Markin, A.V. Determination of methotrexate in spiked human urine using SERS-active sorbent. Anal Bioanal Chem 412, 7757–7766 (2020). https://doi.org/10.1007/s00216-020-02932-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02932-x