Abstract
Purpose
To explore the use of a multistate repeated, time-to-categorical event model describing the frequency, severity and duration of migraines.
Methods
Subject level data from patients in placebo arms from two efficacy trials for migraine-preventive treatments were used. Models were developed using NONMEM 7.3. A survival model was combined with an ordered categorical model to form the repeated-time-to-start of categorical migraine event model, which simultaneously described the time-to-start of migraines and the severity of the starting migraine event. This was linked to a repeated-time-to-end of migraine event model with different hazard functions depending on the severity of the ongoing migraine event. Model performance was internally and externally qualified.
Results
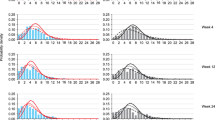
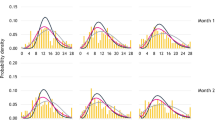
The successfully qualified model showed that patients responding to placebo had a reduction in migraine incidence rate, and a decreased proportion of severe migraines. There was an increase in moderate migraine duration, an increased proportion of mild migraines and a reduction in proportion of severe migraines. Age was related to migraine duration.
Conclusions
The model represents an innovative framework for clinical trial modeling and simulation, and successfully describes placebo effect in migraine prevention. This approach can be adapted to investigate exposure-response relationship of drugs and can also be implemented in other therapeutic areas where the rate, duration and severity of disease episodes are relevant to trial outcomes.





Similar content being viewed by others
Abbreviations
- RTTCE:
-
Repeated time-to-categorical event
- VPC:
-
Visual predictive check
References
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of Disease study 2015. Lancet. 2016;388(10053):1545–602.
Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48(4):553–63.
Headache Classification Committee of the International Headache S. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine Prevalence and prevention (AMPP) study. Headache. 2013;53(8):1278–99.
Charles A. Migraine. N Engl J Med. 2017;377(17):1698–9.
Yuan H, White CS, Silberstein SD. Calcitonin gene-related peptide antagonists in the treatment of episodic migraine. Clin Pharmacol Ther. 2019;105(5):1121–9.
Urits I, Jones MR, Gress K, Charipova K, Fiocchi J, Kaye AD, et al. CGRP antagonists for the treatment of chronic migraines: a comprehensive review. Curr Pain Headache Rep. 2019;23(5):29.
Guidance for Industry. Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. In: USFaD A, editor. ; 1998.
Workgroup EM, Marshall SF, Burghaus R, Cosson V, Cheung SY, Chenel M, et al. Good practices in model-informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst Pharmacol. 2016;5(3):93–122.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model evaluation Group of the International Society of Pharmacometrics best practice C. model evaluation of continuous data Pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):87–109.
Holford N. A time to event tutorial for pharmacometricians. CPT Pharmacometrics Syst Pharmacol. 2013;2:e43.
Plan EL. Modeling and simulation of count data. CPT Pharmacometrics Syst Pharmacol. 2014;3:e129.
Staab A, Tillmann C, Forgue ST, Mackie A, Allerheiligen SR, Rapado J, et al. Population dose-response model for tadalafil in the treatment of male erectile dysfunction. Pharm Res. 2004;21(8):1463–70.
Schindler E, Karlsson MO. A minimal continuous-time Markov Pharmacometric model. AAPS J. 2017;19(5):1424–35.
Ueckert S. Modeling composite assessment data using item response theory. CPT Pharmacometrics Syst Pharmacol. 2018;7(4):205–18.
Bjornsson MA, Simonsson US. Modelling of pain intensity and informative dropout in a dental pain model after naproxcinod, naproxen and placebo administration. Br J Clin Pharmacol. 2011;71(6):899–906.
Hu C, Sale ME. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn. 2003;30(1):83–103.
Pilla Reddy V, Kozielska M, Johnson M, Mafirakureva N, Vermeulen A, Liu J, et al. Population pharmacokinetic-pharmacodynamic modeling of haloperidol in patients with schizophrenia using positive and negative syndrome rating scale. J Clin Psychopharmacol. 2013;33(6):731–9.
Plan EL, Karlsson KE, Karlsson MO. Approaches to simultaneous analysis of frequency and severity of symptoms. Clin Pharmacol Ther. 2010;88(2):255–9.
Beal SS LB.; Boeckmann, A.; Bauer, RJ. NONMEM user’s guide (1989–2009). In.; 2009.
Lindbom L, Pihlgren P, Jonsson EN. PsN-toolkit--a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed. 2005;79(3):241–57.
Kelman L. Migraine changes with age: IMPACT on migraine classification. Headache. 2006;46(7):1161–71.
Perez-Pitarch A, Nock V, Huennemeyer A, Soleymanlou N, Kaspers S, Freijer J. Empagliflozin as Adjunct to Insulin in Patients with Type 1 Diabetes Mellitus: Modelling Rate and Severity of Hypoglycemic Events. In: PAGE meeting. Lisbon, Portugal; 2016.
Cox EH, Veyrat-Follet C, Beal SL, Fuseau E, Kenkare S, Sheiner LB. A population pharmacokinetic-pharmacodynamic analysis of repeated measures time-to-event pharmacodynamic responses: the antiemetic effect of ondansetron. J Pharmacokinet Biopharm. 1999;27(6):625–44.
Juul RV, Rasmussen S, Kreilgaard M, Christrup LL, Simonsson US, Lund TM. Repeated time-to-event analysis of consecutive analgesic events in postoperative pain. Anesthesiology. 2015;123(6):1411–9.
Plan EL, Ma G, Nagard M, Jensen J, Karlsson MO. Transient lower esophageal sphincter relaxation pharmacokinetic-pharmacodynamic modeling: count model and repeated time-to-event model. J Pharmacol Exp Ther. 2011;339(3):878–85.
Karlsson MO, Schoemaker RC, Kemp B, Cohen AF, van Gerven JM, Tuk B, et al. A pharmacodynamic Markov mixed-effects model for the effect of temazepam on sleep. Clin Pharmacol Ther. 2000;68(2):175–88.
Lacroix BD, Karlsson MO, Friberg LE. Simultaneous exposure-response modeling of ACR20, ACR50, and ACR70 improvement scores in rheumatoid arthritis patients treated with Certolizumab Pegol. CPT Pharmacometrics Syst Pharmacol. 2014;3:e143.
Pinana JL, Perez-Pitarch A, Guglieri-Lopez B, Gimenez E, Hernandez-Boluda JC, Terol MJ, et al. Sirolimus exposure and the occurrence of cytomegalovirus DNAemia after allogeneic hematopoietic stem cell transplantation. Am J Transplant. 2018;18(12):2885–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Perez-Pitarch, A., Gottipati, G., Uppoor, R. et al. An Innovative Pharmacometric Approach for the Simultaneous Analysis of Frequency, Duration and Severity of Migraine Events. Pharm Res 37, 189 (2020). https://doi.org/10.1007/s11095-020-02907-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02907-8