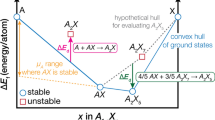
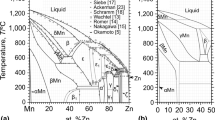
The thermodynamic assessment of the Mg–Ni–Si system has been developed in the framework of the CALPHAD method. The critical review of the literature data has been provided. A set of selfconsistent thermodynamic parameters for the phases in this ternary system is obtained using the phase diagram data available in the literature. The excess Gibbs energy term of solution phases is described using the Redlich–Kister polynomial. The sublattice model is used to describe the MgNi2 intermetallic phase having homogeneity range in the ternary system. Ternary intermetallic compounds are modelled as line compounds. Isothermal sections and liquidus surface, as well as the coordinates of the invariant reactions, are calculated. The calculated results are in satisfactory agreement with the experimental data. The calculated liquidus surface is extremely complex and contains 38 invariant reactions including 8 degenerated reactions and 10 invariant maxima. Most of the presented invariant reactions must be considered as approximate. The τ3 and τ5 phases melt congruently at 1544 and 1487 K, respectively. The τ4 forms via the peritectoid τ5 + NiSi + βNi2Si ↔ τ4 reaction at a temperature of 1193 K. According to our calculations, τ1 and τ8 melt at 1359 K (the L + τ5 ↔ τ1 reaction) and 1267 K (the L + τ1 ↔ τ8 reaction). The τ2 and τ6 are formed via the peritectic reactions at 1317 K (the L + τ3 + MgNi2 ↔ τ2 reaction) and at 1229 K (the L + τ5 + Mg2Si ↔ τ6 reaction), and the τ7 is formed via peritectoid Mg2Si + τ1 + τ5 ↔ τ7 reaction at 1279 K.





Similar content being viewed by others
References
G. Li, H.S. Gill, and R.A. Varin, “Magnesium silicide intermetallic alloys,” Metall. Trans. A., 24, 2383– 2391 (1993).
G.H. Li and R.A. Varin, “Effect of Ni content on the microstructure, microhardness and fracture toughness of magnesium silicide ternary alloy,” Mater. Sci. Eng. A, 183, 145–155 (1994).
R.A. Varin and G.-H. Li, “Microstructure and mechanical properties of Mg33–xSixNi67 intermetallic composite alloys,” Mater. Sci. Engineering A, 192–193, 59–68 (1995).
Y.K. Song and R.A. Varin, “Indentation microcracking and toughness of newly discovered ternary intermetallic phases in the Ni–Si–Mg system,” Intermetallics, 6, 379–393 (1998).
W. Buchholz and H.-U. Schuster, “Intermetallic phases with B35-strtucture and relationship to LiFe6Ge6,” Z. Anorg. Allg. Chem., 482, 40–48 (1981).
G. Hofer and H.H. Stadelmaier, “Cobalt-, Nickel- and Copper-Phases of the MnCu2Al-Type,” Monatsheft. Chemie,” 98, 408–411 (1967).
D. Noréus, L. Eriksson, L. Göthe, P.E. and Werner, “Structure determination of Mg2SiNi3,” J. Less Comm. Metals, 107, 345–349 (1985).
Y.K. Song and R.A. Varin, “New intermetallic phases in the Ni–Si–Mg ternary system,” Intermetallics, 6, 43–59 (1998).
Y.K. Song and R.A. Varin, “Phase equilibria and intermetallic phases in the Ni–Si–Mg ternary system,” Metall. Mat. Trans. A, 32, 5–18 (2001).
M.H.G. Jacobs and P.J. Spencer, “A critical thermodynamic evaluation of the system Mg–Ni,” Calphad, 22, 513–525 (1998).
D. Kevorkov, R. Schmid-Fetzer, and F. Zhang, “Phase equilibria and thermodynamics of the Mg–Si–Li system and remodeling of the Mg–Si system,” J. Phase Equil. Diff., 25, 140–151 (2004).
J.C. Schuster and Y. Du, “Experimental investigation and thermodynamic modeling of the Cr–Ni–Si system,” Metall. Mat. Trans. A, 31, 1795–1803 (2000).
P. Villars and L.D. Calvert, Pearson’s Handbook of Crystallographic Data for Intermetallic Phases, 2nd ed., ASM International, Metals Park, Ohio (1991).
T.B. Massalski and H. Okamoto (Eds.), Binary Alloy Phase Diagrams, ASM International, Materials Park, Ohio (1990).
L. Wang, D. Zhao, J. Lu, W. Liu, Q. Zhou, “Solid-state synthesis and magnetic properties of rhombohedral phase Mg2SiNi3,” Int. J. Mater. Res., 10, No. 9, 177–180 (2018).
K.L. Holman, E. Morosan, P.A. Casey, L. Li, N.P. Ong, T. Klimczuk, C. Felser, R.J. Cava, “Crystal structure and physical properties of Mg6Cu16Si7-type M6Ni16Si7, for M = Mg, Sc, Ti, Nb, and Ta,” Mater. Res. Bull., 43, 9–15 (2008).
A.T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, 317–425 (1991).
O. Dovbenko, L. Dreval, Y. Du, Y. Liu, S. Liu, S. Iljenko, G. Effenberg, “Critical evaluation of ternary phase diagram data: Important considerations in the scrutiny of the correctness, coherence, and interpretation,” Calphad, 68 (2020).
F. Laves and H. Witte, “Influence of valence electron to crystal structure of ternary magnesium alloys,” Metallwirtsch, 15, 840–842 (1936).
F. Laves, “Crystallography of the alloys,” Naturwissensch, 27, 65–73 (1939).
M.Y. Teslyuk, Intermetallic Compounds with Structure of Laves Phases [in Russian], Nauka, Moscow (1969).
Y. Imai, H. Sugawara, Y. Mori, S. Nakamura, A. Yamamoto, K.I. Takarabe, “Energetic consideration of compounds at Mg2Si–Ni electrode interlayer produced by spark-plasma sintering,” Jpn. J. Appl. Phys.,56, 05DC03 (2017).
G. Inden, “Determination of chemical and magnetic interchange energies in B.C.C. alloys. III. Application to ferromagnetic alloys,” Z. Metallkd., 68, 529–534 (1977).
M. Hillert and M. Jarl, “A model for alloying in ferromagnetic metals,” Calphad, 2, 227–238 (1978).
N. Saunders and A.P. Miodownik, Pergamon Materials Series, CALPHAD: Calculation of Phase Diagrams: A Comprehensive Guide, Vol. 1, Pergamon, New York, London (1998), p. 496.
B. Sundman and J. Ågren, “A regular solution model for phases with several components and sublattices, suitable for computer applications,” J. Phys. Chem. Solids., 42, 297–301 (1981).
B. Sundman, B. Jansson, and J.-O. Andersson, “The Thermo-Calc databank system,” Calphad, 9, 153–190 (1985).
Acknowledgements
The financial support from National Natural Science Foundation of China (Grant no. 51820105001) is acknowledged. Both Dr. Oleksandr Dovbenko and Dr. Liya Dreval thank the grant from Department of International Cooperation and Exchanges of Central South University, China for their visit to Central South University from September 1, 2018 to November 30, 2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Poroshkova Metallurgiya, Vol. 59, Nos. 3–4 (532), pp. 120–137, 2020.
Rights and permissions
About this article
Cite this article
Zeng, Y., Dreval, L., Dovbenko, O. et al. Thermodynamic Assessment of the Mg–Ni–Si System. Powder Metall Met Ceram 59, 209–223 (2020). https://doi.org/10.1007/s11106-020-00153-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00153-6