Abstract
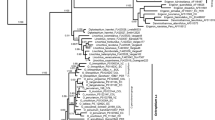
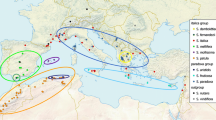
The Andes is recognized as one of the most biodiverse places on Earth, promoting in its uplift process a series of recent rapid diversification events in different biotic groups like birds, mammals, insects and vascular plants. The uplift of the Andes during the Cenozoic acted as a barrier for many biotic groups, as a scenario for radiation processes due to occupancy of different niches and as a corridor for others. Connections between the Andes and the Atlantic Forest showed intermittent phases along the Cenozoic, affecting the distribution patterns and diversification of different biotic groups. Nowadays, the Andes and the Atlantic Forest are both considered globally relevant biodiversity hotspots. Floristic groups thriving in both hotspots are crucial for a better understanding of their biogeographic history, as well as for informing future conservation actions. Mutisia (Asteraceae), a genus comprising 63 perennial shrubs and vines endemic to South America, shows a marked West-East disjunction: Most species occupy almost the whole Andean chain from Colombia to Patagonia, while a second group encompasses four species distributed in eastern Brazil and the surrounding areas of Paraguay, Uruguay and Argentina. We reconstructed the phylogeny of the genus to assess its possible biogeographic history. We analysed three DNA regions, i.e. the chloroplast trnL-trnF intergenic spacer and the nuclear ribosomal internal and external transcribed spacers, ITS and ETS. Using maximum likelihood and Bayesian inference, gene trees were reconstructed, and a concatenated phylogenetic tree was inferred. Divergence times were estimated by means of BEAST, and the ancestral areas were inferred using BioGeoBEARS. An ancestral reconstruction of morphological traits was also performed, as well as maps representing current richness hotspots within the genus. Phylogenetic analyses strongly support the monophyly of Mutisia, with two well-supported main clades: clade A, with presence of Atlantic-central-northern Andes species, and clade B, with central/southern Andes species. Dating analyses suggest that a main clade separation occurred at the early Miocene, followed by the separation of the Atlantic clade A2 by the late Miocene, and more recent radiations occurred in the central, northern and southern Andes during the Pliocene. Results are in tune with other angiosperm taxa that also underwent rapid radiations, possibly related to environmental and pollinator changes. The biogeographic history of Mutisia is related to morphological adaptations, history and geographic factors acting since the Miocene along the Andes and adjacent areas. Threat assessments and conservation actions for the genus shall include the whole distribution range, including low-range northern and southern Andes species, as well as the distinctive Atlantic Forest clade.






Similar content being viewed by others
References
Abrahamczyk, S., & Renner, S. S. (2015). The temporal build-up of hummingbird/plant mutualisms in North America and temperate South America. BMC Evolutionary Biology, 15(1), 1–12. https://doi.org/10.1186/s12862-015-0388-z.
Achimón, F., Johnson, L. A., Cocucci, A. A., Sérsic, A. N., & Baranzelli, M. C. (2018). Species tree phylogeny, character evolution, and biogeography of the Patagonian genus Anarthrophyllum Benth. (Fabaceae). Organisms, Diversity and Evolution, 18, 71–86.
Albert, J. S., Lovejoy, N. R., & Crampton, W. G. R. (2006). Miocene tectonism and the separation of cis- and trans-Andean river basins: evidence from Neotropical fishes. Journal of South American Earth Sciences, 21, 14–27.
Antonelli, A., Kissling, W. D., Flantua, S. G. A., Bermúdez, M. A., Mulch, A., Muellner-Riehl, A. N., Kreft, H., Linder, H. P., Badgley, C., Fjeldså, J., Fritz, S. A., Rahbek, C., Herman, F., Hooghiemstra, H., & Hoorn, C. (2018). Geological and climatic influences on mountain biodiversity. Nature Geoscience, 11, 718–725.
Antonelli, A., Nylander, J. A. A., Persson, C., & Sanmartín, I. (2009). Tracing the impact of the Andean uplift on Neotropical plant evolution. PNAS, 106, 9749–9754.
Armijo, R., Lacassin, R., Coudurier-Curveur, A., & Carrizo, D. (2015). Coupled tectonic evolution of Andean orogeny and global climate. Earth Science Reviews, 143, 1–35.
Arroyo, M. T. K., Primack, R., & Armesto, J. (1982). Community studies in pollination ecology in the high temperate Andes of Central Chile I: pollination mechanisms and altitudinal variation. American Journal of Botany, 69, 82–97.
Baird, K. E., Funk, V. A., Wen, J., & Weeks, A. (2010). Molecular phylogenetic analysis of Leibnitzia Cass. (Asteraceae: Mutisieae: Gerbera-complex), an Asian-North American disjunct genus. Journal of Systematics and Evolution, 48, 161–174.
Baldwin, B. G., & Markos, S. (1998). Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Molecular Phylogenetics and Evolution, 10, 449–463.
Barnes, J. B., Ehlers, T. A., Insel, N., Mcquarrie, N. & Poulsen, C. J. (2012). Linking orography, climate, and exhumation across the central Andes. Geology, 40, 1135–1138.
Barreda, V. D., Palazzesi, L., Tellería, M. C., Katinas, L., Crisci, J. V., Bremer, K., Passalia, M. G., Corsolini, R., Rodríguez Brizuela, R., & Bechis, F. (2010). Eocene Patagonia fossils of the daisy family. Science, 329(5999), 1621.
Barreda, V., Palazzesi, L., Katinas, L., Crisci, J., Tellería, M., Bremer, K., Passala, M.G., Bechis, F. & Corsolini, R. (2012). An extinct Eocene taxon of the daisy family (Asteraceae): evolutionary, ecological and biogeographical implications. Annals of Botany, 109, 127–134.
Barreda, V. D., Palazzesi, L., Tellería, M. C., Olivero, E. B., Raine, J. I., & Forest, F. (2015). Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica. PNAS, 112, 10989–10994.
Barreda, V. D., Palazzesi, L., Tellería, M. C., Olivero, E. B., Raine, J. I., & Forest, F. (2016). Reply to Panero: Robust phylogenetic placement of fossil pollen grains: the case of Asteraceae. PNAS, 113, E412.
Bell, M. A., & Lloyd, G. T. (2014). Strap: Stratigraphic Tree Analysis for Palaeontology. https://CRAN.R-project.org/package=strap
Bollback, J. P. (2006). SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics, 7, 88. https://doi.org/10.1186/1471-2105-7-88.
Bonifacino, J. M., & Funk, V. A. (2012). Phylogenetics of the Chiliotrichum group (Compositae: Astereae): the story of the fascinating radiation in the paleate Astereae genera from southern South America. Taxon, 61, 180–196.
Bowman, D. M. J. S., Moreira-Muñoz, A., Kolden, C. A., Chávez, R. O., Muñoz, A. A., Salinas, F., González-Reyes, Á., Rocco, R., de la Barrera, F., Williamson, G. J., Borchers, N., Cifuentes, L. A., Abatzoglou, J. T., & Johnston, F. H. (2019). Human-environmental drivers and impacts of the globally extreme 2017 Chilean fires. Ambio, 48, 350–362.
Brown, J. L. (2014). SDMtoolbox: a python-based toolkit for landscape genetic, biogeography, and species distribution model analyses. Methods in Ecology and Evolution, 5, 694–700.
Brown, J. L., Bennett, J. R., & French, C. M. (2017). SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 5:e4095. https://doi.org/10.7717/peerj.4095.
Brumfield, R. T., & Edwards, S. V. (2007). Evolution into and out of the Andes: a Bayesian analysis of historical diversification in Thamnophilus antshrikes. Evolution, 61, 346–367. https://doi.org/10.1111/j.1558-5646.2007.00039.x.
Buzato, S., Sazima, M., & Sazima, I. (2000). Hummingbird-pollinated floras at three Atlantic Forest sites. Biotropica, 32, 824–841.
Cabanne, G. S., Campagna, L., Trujillo-Arias, N., Naoki, K., Gómez, I., Miyaki, C. Y., Santos, F. R., Dantas, G. P. M., Aleixo, A., Claramunt, S., Rocha, A., Caparroz, R., Lovette, I. J., & Tubaro, P. L. (2019). Phylogeographic variation within the buff-browed foliage-gleaner (Aves: Furnariidae: Syndactyla rufosuperciliata) supports an Andean-Atlantic forests connection via the Cerrado. Molecular Phylogenetics and Evolution, 133, 198–213.
Cabrera, A. (1965). Revisión del género Mutisia (Compositae). Opera Lilloana, 13, 1–227.
Carrapa, B., Clementz, M., & Feng, R. (2019). Ecological and hydroclimate responses to strengthening of the Hadley circulation in South America during the late Miocene cooling. PNAS, 116, 9747–9752.
Chacón, J., Camargo de Assis, M., Meerow, A. W., & Renner, S. S. (2012). From East Gondwana to Central America: historical biogeography of the Alstroemeriaceae. Journal of Biogeography, 39, 1806–1818.
Chaves, J. A., Weir, J. T., & Smith, T. B. (2011). Diversification in Adelomyia hummingbirds follows Andean uplift. Molecular Ecology, 20(21), 4564–4576. https://doi.org/10.1111/j.1365-294X.2011.05304.x.
Chazot, N., Freitas, A. V. L., De-Silva, D. L., Willmott, K. R., Lamas, G., Mallet, J., et al. (2018). Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies. Ecology and Evolution, 8, 3965–3982.
Contreras-Ortiz, N., Atchison, G. W., Hughes, C. E., & Madriñan, S. (2018). Convergent evolution of high elevation plant growth forms and geographically structured variation in Andean Lupinus (Fabaceae). Botanical Journal of the Linnean Society, 187, 118–136.
Costa, L. (2003). The historical bridge between the Amazon and the Atlantic Forest of Brazil: a study of molecular phylogeography with small mammals. Journal of Biogeography, 30, 71–86.
da Silva Tomadon, L., Dettke, G. A., Galeazzi Caxambu, M., Malfetoni Ferreira, J. I., & do Couto, E. V. (2019). Significance of forest fragments for conservation of endangered vascular plant species in southern Brazil hotspots. Écoscience, 26, 221–235.
Damascos, M. A., Barthelemy, D., Ezcurra, C., Martínez, P., & Brion, C. (2008). Phenology, shoot growth, and branching pattern in Mulinum spinosum (Apiaceae), a cushion shrub of the arid Patagonian steppe of Argentina. Journal of Arid Environments, 72, 1977–1988.
De la Riva, I., García-París, M., & Parra-Olea, G. (2010). Systematics of Bolivian frogs of the genus Telmatobius (Anura, Ceratophryidae) based on mtDNA sequences. Systematics and Biodiversity, 8, 49–61.
Diazgranados, M., & Barber, J. C. (2017). Geography shapes the phylogeny of frailejones (Espeletiinae Cuatrec., Asteraceae): a remarkable example of recent rapid radiation in sky islands. PeerJ, 5(e2968), 1–35.
Drummond, A. J., & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214. https://doi.org/10.1186/1471-2148-7-214.
Ehlers, T. A., & Poulsen, C. J. (2009). In fluence of Andean uplift on climate and paleoaltimetry estimates. Earth and Planetary Science Letters, 281, 238–248.
Fiorella, R., Poulsen, C. J., Pillco Zolá, R. S., Jeffery, M. L., & Ehlers, T. A. (2015). Modern and long-term evaporation of central Andes surface waters suggests paleo archives underestimate Neogene elevations. Earth and Planetary Science Letters, 432, 59–72.
Flantua, S. G. A., O'Dea, A., Onstein, R. E., Giraldo, C., & Hooghiemstra, H. (2019). The flickering connectivity system of the north Andean páramos. Journal of Biogeography, 46, 1808–1825.
Funk, V. A., Sancho, G., Roque, N. d., Kelloff, C. L., Ventosa-Rodríguez, I., Diazgranados, M., Bonifacino, M, & Chan, R. (2014). A phylogeny of the Gochnatieae: understanding a critically placed tribe in the Compositae. Taxon, 63(4), 859–882.
Garzione, C. N., Mcquarrie, N., Perez, N. D., Ehlers, T. A., Beck, S. L., Kar, N., et al. (2017). Tectonic evolution of the Central Andean Plateau and implications for the growth of plateaus. Annual Review of Earth and Planetary Sciences, 45, 529–559.
Gregory-Wodzicki, K. M. (2000). Uplift history of the central and northern Andes: a review. Geological Society of America Bulletin, 112, 1091–1105.
Grimmer, F., Dupont, L., Lamy, F., Jung, G., González, C., & Wefer, G. (2018). Early Pliocene vegetation and hydrology changes in western equatorial South America. Climate of the Past, 14, 1739–1754.
Grossi, M., Draper, D., Apodaca, M., Vitali, M., Pataro, L., Katinas, L., & Moreno Saiz, J. (2017). The road to 2020 targets and the learnings from the emblematic south American plant genus Nassauvia (Asteraceae). Biodiversity and Conservation, 26, 329–351.
Gruenstaeudl, M., Urtubey, E., Jansen, R. K., Samuel, R., Barfuss, M. H. J., & Stuessy, T. F. (2009). Phylogeny of Barnadesioideae (Asteraceae) inferred from DNA sequence data and morphology. Molecular Phylogenetics and Evolution, 51, 572–587.
Hazzi, N. A., Sebastián, J., Ortiz-Movliav, C., & Darío, R. (2018). Biogeographic regions and events of isolation and diversification of the endemic biota of the tropical Andes. PNAS, 115(31), 7985–7990.
Heibl, C. (2008, Onwards). PHYLOCH: R language tree plotting tools and interfaces to diverse phylogenetic software packages. http://www.christophheibl.de/Rpackages.html
Hershkovitz, M. A., Arroyo, M. T. K., Bell, C., & Hinojosa, L. F. (2006). Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both ancient and recent origins of the high elevation lineages. Molecular Phylogenetics and Evolution, 41, 594–605.
Hoorn, C., Wesselingh, F. P., Hovikoski, J., & Guerrero, J. (2010a). The development of the Amazonian mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia). In C. Hoorn & F. P. Wesselingh (Eds.), Amazonia, landscape and species evolution: a look into the past (1st ed., pp. 123–142). Oxford: Blackwell Publishing.
Hoorn, C., Wesselingh, F., & Steege, H. (2010b). Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science, 330, 927–931.
Hoorn, C., Perrigo, A., & Antonelli, A. (2018). Mountains, climate and biodiversity: An introduction. In C. Hoorn, A. Perrigo, & A. Antonelli (Eds.), Mountains, climate and biodiversity (pp. 1–13). Oxford: Wiley Blackwell.
Jara-Arancio, P., Vidal, P. M., & Arroyo, M. T. K. (2018). Phylogenetic reconstruction of the genus Triptilion (Asteraceae, Nassauvieae) based on nuclear and chloroplast DNA sequences. Journal of Systematics and Evolution, 56(2), 120–128. https://doi.org/10.1111/jse.12294
Jara-Arancio, P., Vidal, P. M., Panero, J. L., Marticorena, A., Arancio, G., & Arroyo, M. T. K. (2017). Phylogenetic reconstruction of the South American genus Leucheria Lag. (Asteraceae, Nassauvieae) based on nuclear and chloroplast DNA sequences. Plant Systematics and Evolution, 303, 221–232.
Katinas, L., Pruski, J., Sancho, G., & Tellería, M. C. (2008a). The subfamily Mutisioideae (Asteraceae). Botanical Review, 74, 469–716.
Katinas, L., Crisci, J. V., Jabaily, R. S., Williams, C., Walker, J., Drew, B., Bonifacino, J. M., & Sytsma, K. J. (2008b). Evolution of secondary heads in Nassauviinae (Asteraceae, Mutisieae). American Journal of Botany, 95, 229–240.
Katinas, L., Sancho, G., Tellería, M. C., & Crisci, J. V. (2009). Mutisieae sensu stricto (Mutisioideae sensu stricto). In Funk, V., Susanna, A., Stuessy, T. F., & Bayer, R.J. (Eds.) Systematics, Evolution, and Biogeography of Compositae, IAPT editor, chap. 14, pp. 229–248.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability article fast track. Molecular Biology and Evolution, 30, 772–780.
Lamb, S. (2016). Cenozoic uplift of the central Andes in northern Chile and Bolivia —reconciling paleoaltimetry with the geological evolution. Canadian Journal of Earth Sciences, 53, 1227–1245.
Ledo, R. M. D., & Colli, G. R. (2017). The historical connections between the Amazon and the Atlantic Forest revisited. Journal of Biogeography, 44, 2551–2563.
Luebert, F., & Weigend, M. (2014). Phylogenetic insights into Andean plant diversification. Frontiers in Ecology and Evolution, 2, 1–17.
Luebert, F., & Pliscoff, P. (2017). Sinopsis bioclimática y vegetacional de Chile. Editorial Universitaria (2nd ed.). Chile: Santiago.
Luebert, F., Wen, J., & Dillon, M. O. (2009). Systematic placement and biogeographical relationships of the monotypic genera Gypothamnium and Oxyphyllum (Asteraceae: Mutisioideae) from the Atacama Desert. Botanical Journal of the Linnean Society, 159, 32–51.
Luebert, F., Lörch, M., Acuña, R., Mello-Silva, R., Weigend, M., & Mutke, J. (2020). Clade-specific biogeographic history and climatic niche shifts of the southern Andean-southern Brazilian disjunction in plants. In V. Rull & A. C. Carnaval (Eds.), Neotropical diversification: patterns and processes (pp. 661–682). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-31167-4_24.
Maddison, W. P., & Maddison, D.R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.51. http://www.mesquiteproject.org.
Madriñán, S., Cortés, A. J., & Richardson, J. E. (2013). Páramo is the world’ s fastest evolving and coolest biodiversity hotspot. Frontiers in Genetics, 4, 1–7.
Mansilla, P., Panez Pinto, A., & Ponce-Hille, M. I. (2019). Discursos geopolíticos de desarrollo y reestructuración territorial IIRSA en el eje Mercosur-Chile. Dialogo Andino, 59, 37–54.
Mairal, M., Sanmartín, I., & Pellissier, L. (2017). Lineage-specific climatic niche drives the tempo of vicariance in the Rand Flora. Journal of Biogeography, 44(4), 911–923. https://doi.org/10.1111/jbi.12930.
Markos, S., & Baldwin, B. G. (2001). Higher-level relationships and major lineages of Lessingia (Compositae, Astereae) based on nuclear rDNA internal and external transcribed spacer (ITS and ETS) sequences. Systematic Botany, 26, 168–183.
Marquínez, X., Lohmann, L. G., Salatino, M. L. F., Salatino, A., & González, F. (2009). Generic relationships and dating of lineages in Winteraceae based on nuclear (ITS) and plastid (rpS16 and psbA-trnH) sequence data. Molecular Phylogenetics and Evolution, 53(2), 435–449. https://doi.org/10.1016/j.ympev.2009.07.001.
Martínez, C., Jaramillo, C., Correa-Metrío, A., Crepet, W., Moreno, J., Aliaga, A., et al. (2020). Neogene precipitation, vegetation and elevation history of the Central Andean Plateau. Science Advances, in press.
Matzke, N. J. (2013). Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Frontiers of Biogeography, 5, 242–248. http://escholarship.org/uc/item/44j7n141.
Matzke, N. J. (2014). Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Systematic Biology, 63, 951–970.
McGuire, J. A., Witt, C. C., Remsen Jr., J. V., Corl, A., Rabosky, D. L., Altshuler, D. L., & Dudley, R. (2014). Molecular phylogenetics and the diversification of hummingbirds. Current Biology, 24(8), 910–916. https://doi.org/10.1016/j.cub.2014.03.016.
Miller, M. A., Pfeiffer, W., & Schwartz, T. (2010). Creating the CIPRES science gateway for inference of large phylogenetic trees. Gateway Computing IEEExplore. https://doi.org/10.1109/GCE.2010.5676129.
Mittermeier, R. A., Turner, W. R., & Larsen, F. W. (2011). Global biodiversity conservation: the critical role of hotspots. In F. E. Zachos & J. C. Habel (Eds.), Biodiversity hotspots: distribution and protection of conservation priority areas (pp. 3–22). Berlin Heidelberg: Springer.
Molotoks, A., Kuhnert, M., Dawson, T., & Smith, P. (2017). Global hotspots of conflict risk between food security and biodiversity conservation. Land, 67, 1–15.
Monge, M. (2011). As tribos Barnadesieae e Mutisieae s.l. (Asteraceae) no estado de São Paulo, Brasil. Dissertação de Mestrado, Universidade Estadual de Campinas, pp. 1–227.
Monge, M., & Semir, J. (2020). Mutisia in Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. Resource Document: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB27298
Moreira-Muñoz, A. (2011). Plant geography of Chile. Dordrecht: Springer Netherlands.
Moreira-Muñoz, A. (2014). Central Chile Ecoregion. In C. Hobohm (Ed.), Endemism in Vascular Plants (pp. 221–233). Dordrecht: Springer.
Moreira-Muñoz, A., Morales, V., & Muñoz-Schick, M. (2012). Actualización sistemática y distribución geográfica de Mutisioideae (Asteraceae) de Chile. Gayana Botánica, 69, 9–29.
Nicola, M. V., Johnson, L. A., & Pozner, R. (2019). Molecular phylogenetics and evolution unraveling patterns and processes of diversification in the south Andean- Patagonian Nassauvia subgenus Strongyloma (Asteraceae, Nassauvieae). Molecular Phylogenetics and Evolution, 136, 164–182.
Oliveira-Filho, A. T., Jarenkow, J. A., & Nogueira Rodal, M. J. (2006). Floristic relationships of seasonally dry forests of eastern South America based on tree species distribution patterns. In Pennington, T. R., Lewis, G. P. & ratter J. A. (Eds.) Neotropical savannas and dry forests: Diversity, biogeography, and conservation. CRC press, pp. 151-184.
Ortiz, S., Bonifacino, J. M., Crisci, J. V, Funk, V. A., Hansen, H. V, Hind, D. J. N., et al. (2009). The basal grade of Compositae: Mutisieae (sensu Cabrera) and Carduoideae. In V. A. Funk, A. Sussana, T. F. Stuessy, & R. Bayer (Eds.), Systematics, Evolution and Biogeography of Compositae (1st ed., pp. 193–213).
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877–884.
Palazzesi, L., Gottschling, M., Barreda, V., & Weigend, M. (2012). First Miocene fossils of Vivianiaceae shed new light on phylogeny, divergence times, and historical biogeography of Geraniales. Biological Journal of the Linnean Society, 107(1), 67–85. https://doi.org/10.1111/j.1095-8312.2012.01910.x.
Panero, J. L. (2016). Phylogenetic uncertainty and fossil calibration of Asteraceae chronograms. PNAS, 113, E411.
Panero, J. L., & Crozier, B. S. (2016). Molecular phylogenetics and evolution macroevolutionary dynamics in the early diversification of Asteraceae. Molecular Phylogenetics and Evolution, 99, 116–132.
Panero, J. L., & Funk, V. A. (2008). The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Molecular Phylogenetics and Evolution, 47, 757–782.
Paradis, E., Claude, J., & Strimmer, K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290.
Paradis, E., & Schliep, K. (2019). Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics, 35, 526–528.
Pasini, E., Funk, V. A., de Souza-Chies, T. T., & Miotto, S. T. S. (2016). New insights into the phylogeny and biogeography of the Gerbera-complex (Asteraceae: Mutisieae). Taxon, 65, 547–562.
Pelser, P. B., Kennedy, A. H., Tepe, E. J., Shidler, J. B., Nordenstam, B., Kadereit, J. W., & Watson, L. E. (2010). Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. American Journal of Botany, 97, 856–873.
Pennell, M. W., Eastman, J. M., Slater, G. J., Brown, J. W., Uyeda, J. C., Fitzjohn, R. G., Alfaro, M. E., & Harmon, L. J. (2014). Geiger 2.0: and expanded suite of methods for fitting macroevolutionary models to phylogenetics trees. Bioinformatics, 30, 2216–2218.
Pokorny, L., Riina, R., Mairal, M., Meseguer, A. S., Culshaw, V., Cendoya, J., Serrano, M., Carbajal, R., Ortiz, S., Heuertz, M., & Sanmartín, I. (2015). Living on the edge: timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Frontiers in Genetics, 6, 154. https://doi.org/10.3389/fgene.2015.00154.
Pouchon, C., Fernández, A., Nassar, J. M., Boyer, F., Aubert, S., Lavergne, S., & Mavárez, J. (2018). Phylogenomic analysis of the explosive adaptive radiation of the Espeletia complex (Asteraceae) in the Tropical Andes. Systematic Biology, 67(6), 1041–1060.
Prates, I., Melo-Sampaio, P. R., de Oliveira Drummond, L., Teixeira Jr., M., Trefaut Rodrigues, M., & Carnaval, A. C. (2017). Biogeographic links between southern Atlantic Forest and western South America: rediscovery, re-description, and phylogenetic relationships of two rare montane anole lizards from Brazil. Molecular Phylogenetics and Evolution, 113, 49–58.
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using tracer 1.7. Systematic Biology, 67, 901–904.
Ree, R., & Smith, S. (2008). Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology, 57, 4–14.
Ree, R. H., & Sanmartín, I. (2018). Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. Journal of Biogeography, 45, 741–749.
Reginato, M., & Michelangeli, F. A. (2016). Diversity and constraints in the floral morphological evolution of Leandra s. str. (Melastomataceae). Annals of Botany, 118, 445–458.
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Revell, L. J., Harmon, L. J., & Collar, D. C. (2008). Phylogenetic signal, evolutionary process, and rate. Systematic Biology, 57, 591–601.
Rohrmann, A., Sachse, D., Mulch, A., Pingel, H., Tofelde, S., Alonso, R. N., & Strecker, M. R. (2016). Miocene orographic uplift forces rapid hydrological change in the southern central Andes. Scientific Reports, 6, 1–7.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Sancho, G., Katinas, L., Viera Barreto, J. N., Moreira-Muñoz, A., & Luebert, F. (2018). Phylogenetic relationships and generic reassessment of Proustia and allies (Compositae: Nassauvieae). Taxon, 67, 113–129.
Särkinen, T., Pennington, R. T., Lavin, M., Simon, M. F., & Hughes, C. E. (2012). Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andean dry tropical forests. Journal of Biogeography, 39, 884–900.
Scherson, R. A., Vidal, R., & Sanderson, M. J. (2008). Phylogeny, biogeography, and rates of diversification of New World Astragalus (Leguminosae) with an emphasis on South American radiations. American Journal of Botany, 95, 1030–1039.
Serrano-Serrano, M. L., Rolland, J., Clark, J. L., Salamin, N., & Perret, M. (2017). Hummingbird pollination and the diversification of angiosperms: and old and successful association in Gesneriaceae. Proceedings of the Royal Society B, 284, 20162816.
Simpson, B. B. (2014). Whence the flora of southern South America? Biases may distort our conclusions. In W. D. Stevens, O. M. Montiel, & P. H. Raven (Eds.), Paleobotany and biogeography: a Festschrift for Alan Graham in his 80th year (pp. 338–366). St. Louis: Missouri Botanical Garden Press.
Simpson, B. B., Arroyo, M. T. K., Sipe, S., Dias de Moraes, M., & McDill, J. (2009). Phylogeny and evolution of Perezia (Asteraceae: Mutisieae: Nassauviinae). Journal of Systematics and Evolution, 47, 431–443.
Sleumer, H. O. (1980). Flacourtiaceae. Flora Neotropica (Vol. 22). New York: New York Botanical Garden Press.
Stamatakis, A. (2014). RaxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313.
Strelin, M. M., Arroyo, J. I., Fliesswasser, S., & Ackermann, M. (2017). Diversification of Caiophora (Loasaceae subfam. Loasoideae) during the uplift of the central Andes. Organisms, Diversity and Evolution, 17, 29–41.
Ströher, P. R., Meyer, A. L. S., Zarza, E., Tsai, W. L. E., McCormack, J. E., & Pie, M. R. (2019). Phylogeography of ants from the Brazilian Atlantic Forest. Organisms, Diversity and Evolution, 19, 435–445.
Stuessy, T. F., Sang, T., & DeVore, M. L. (1996). Phylogeny and biogeography of the subfamily Barnadesioideae with implications for early evolution of the Compositae. In D. J. N. Hind & H. J. Beentje (Eds.), Compositae: Systematics, Proceedings of the International Compositae Conference, Kew, 1994, vol (Vol. 1, pp. 463–490). Kew: Royal Botanic Gardens.
Taberlet, P., Gielly, L., Pautou, G., & Bouvet, J. (1991). Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology, 17, 1105–1109.
Tank, D. C., & Donoghue, M. J. (2010). Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Systematic Botany, 35, 425–441.
Trujillo-Arias, N., Dantas, G. P. M., Arbeláez-Cortés, E., Naoki, K., Gómez, M. I., Santos, F. R., Miyaki, C. Y., Aleixo, A., Tubaro, P. L., & Cabanne, G. S. (2017). The niche and phylogeography of a passerine reveal the history of biological diversification between the Andean and the Atlantic forests. Molecular Phylogenetics and Evolution, 112, 107–121.
Trujillo-Arias, N., Calderón, L., Santos, F. R., Miyaki, C. Y., Aleixo, A., Witt, C. C., Tubaro, P. L., & Cabanne, G. S. (2018). Forest corridors between the central Andes and the southern Atlantic Forest enabled dispersal and peripatric diversification without niche divergence in a passerine. Molecular Phylogenetics and Evolution, 128, 221–232.
Ulloa Ulloa, C. & Jørgensen, P. (1996). A new species of Mutisia (Compositae-Mutisieae) from Ecuador. Novon, 6, 131–133.
Vogel, S. (2015). Vertebrate pollination in Compositae: floral syndromes and field observations. Stapfia, 103, 5–26.
Wagstaff, S. J., & Breitwieser, I. (2002). Phylogenetic relationships of New Zealand Asteraceae inferred from ITS sequences. Plant Systematics and Evolution, 231, 203–224.
Wagstaff, S. J., Breitwieser, I., & Ito, M. (2011). Evolution and biogeography of Pleurophyllum (Astereae, Asteraceae), a small genus of megaherbs endemic to the subantarctic islands. American Journal of Botany, 98, 62–75.
White, T. M., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: a guide to methods and applications (pp. 315–321). San Diego, CA: Academic Press.
Whittaker, R. J., Araújo, M. B., Jepson, P., Ladle, R. J., Watson, J. E. M., & Willis, K. J. (2005). Conservation biogeography: assessment and prospect. Diversity and Distributions, 11, 3–23.
Wilson, S. J., & Coomes, O. T. (2019). ‘Crisis restoration’ in post-frontier tropical environments: Replanting cloud forests in the Ecuadorian Andes. Journal of Rural Studies, 67, 152–165.
Xu, X., Zheng, W., Funk, V. A., & Wen, J. (2018a). Home at Last II: Gerbera hieracioides (Kunth) Zardini (Mutisieae, Asteraceae) is really a Chaptalia. PhytoKeys, 95, 93–106.
Xu, X., Zheng, W., Funk, V. A., Li, K., Zhang, J., & Wen, J. (2018b). Home at last III: Transferring Uechtritzia and Asian Gerbera species into Oreoseris (Compositae, Mutisieae). PhytoKeys, 96, 1–19. https://doi.org/10.3897/phytokeys.96.23142
Zapata, F. (2013). A multilocus phylogenetic analysis of Escallonia (Escalloniaceae): Diversification in montane South America. American Journal of Botany, 100(3), 526–545. https://doi.org/10.3732/ajb.1200297.
Acknowledgments
Several colleagues assisted in the field or with the identification of materials and access to collections. Special thanks to Mélica Muñoz-Schick (SGO), Carla Maldonado and Stephan Beck (both LPB), Katya Romoleroux (QCA Puce), and Maximilian Weigend (BONN). Henry Gonzales, Gustavo Shimizu and Ricardo Jaramillo helped with photos from the species. M. Monge would like to thank Joao Semir, Marcelo Reginato, Carol Devides and Vinicius Brito for support with the analyses.
Funding
This project has received funding from the Chilean National Research Agency (ANID) by means of two grants to AM-M (Fondecyt 1150425 / 1180211). MM acknowledges the grants (PNPD - Capes 001, Fapesp 09/51706-5 & 11/290-3).
Author information
Authors and Affiliations
Contributions
AM-M, RAS, MM, FL conceived the study. AM-M, FL, MM, MD studied and collected exemplars in the field. RAS, MJR, HS prepared and performed material extraction and analyses. FL, AM-M, MM and MD did complementary analyses and prepared all figures. AM-M, RAS, MM, FL drafted the manuscript. All authors have read and approve the final version of this manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreira-Muñoz, A., Scherson, R., Luebert, F. et al. Biogeography, phylogenetic relationships and morphological analyses of the South American genus Mutisia L.f. (Asteraceae) shows early connections of two disjunct biodiversity hotspots. Org Divers Evol 20, 639–656 (2020). https://doi.org/10.1007/s13127-020-00454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-020-00454-z