Abstract
Introduction
Prostatitis is likely to occur in younger or middle-aged men, while prostate cancer is likely to occur in older men. Although amino acids and lipids as biomarkers of prostate cancer have been examined using prostate cancer cell lines/tissues, no previous studies have evaluated amino acids or lipids as potential chronic prostatitis biomarkers.
Objectives
The study’s aim was to identify amino acids and lipids that could serve as potential biomarkers of chronic prostatitis.
Methods
We profiled the amino acids and lipids found in plasma from rats collected in a previous study. In brief, a total of 148 Sprague–Dawley rats (offspring) were dosed with estradiol benzoate (EB) on postnatal days (PNDs) 1, 3 and 5, and subsequently dosed with testosterone (T)/estradiol (E) tubes via subcutaneous implants from PND 90 to 200. Plasma was collected on PNDs 30, 90, 100, 145 and 200. Analysis was conducted with a Xevo TQ-S triple-quadrupole mass spectrometer using a Biocrates AbsoluteIDQ p180 kit.
Results
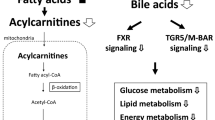
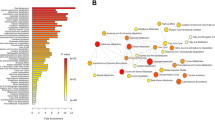
Plasma acylcarnitines [(C2, C16:1, C18, C18:1, C18:1-OH, and C18:2)], glycerophospholipids (lysophosphatidylcholine-acyl, -di-acyl, and -di-acyl acyl-alkyl) and sphingomyelins [SM (OH) C16:1, SM C18:0, SM C18:1, and SM C20:2] significantly increased on PND 145, when chronic inflammation was observed in the dorsolateral prostate of rats dosed with EB, T, and E. No statistical significances of amino acid levels were observed in the EB + T + E group on PND 145.
Conclusion
Exposure to EB, T, and E altered lipid levels in rat plasma with chronic prostate inflammation. These findings suggest that the identified lipids may be predictive chronic prostatitis biomarkers. The results require confirmation through additional nonclinical and human studies.




Similar content being viewed by others
Data availability
The data will be deposited at Metabolights once the manuscript is accepted.
References
Aiyar, N., Disa, J., Ao, Z., Ju, H., Nerurkar, S., Willette, R. N., et al. (2007). Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Molecular and Cellular Biochemistry, 295(1–2), 113–120.
Al-Kadhi, O., Traka, M. H., Melchini, A., Troncoso-Rey, P., Jurkowski, W., Defernez, M., et al. (2017). Increased transcriptional and metabolic capacity for lipid metabolism in the peripheral zone of the prostate may underpin its increased susceptibility to cancer. Oncotarget, 8(49), 84902–84916.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300.
Bi, X., & Henry, C. J. (2017). Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutrition and Diabetes, 7(3), e249. https://doi.org/10.1038/nutd.2016.55.
Butler, L. M., Centenera, M. M., & Swinnen, J. V. (2016). Andrtogen control of lipid metabolism in prostate cancer: Novel insights and future applications. Endocrine-Related Cancer, 23(5), R219–R227. https://doi.org/10.1530/ERC-15-0556.
Collins, M. M., Stafford, R. S., O'Leary, M. P., & Barry, M. J. (1998). How common is prostatitis? A national survey of physician visits. Journal of Urology, 159(4), 1224–1228.
Dereziński, P., Klupczynska, A., Sawicki, W., Pałka, J. A., & Kokot, Z. J. (2017). Amino acid profiles of serum and urine in search for prostate cancer biomarkers: A pilot study. International Journal of Medical Sciences, 14(1), 1–12. https://doi.org/10.7150/ijms.15783.
Doiron, R. C., & Nickel, J. C. (2018). Management of chronic prostatitis/chronic pelvic pain syndrome. Canadian Urological Association Journal, 12(6 Suppl 3), S161–S163. https://doi.org/10.5489/cuaj.5325.
Gilleran, J. P., Putz, O., DeJong, M., DeJong, S., Birch, L., Pu, Y., et al. (2003). The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology, 144(5), 2046–2054.
Gómez-Cebrián, N., Rojas-Benedicto, A., Albors-Vaquer, A., López-Guerrero, J. A., Pineda-Lucena, A., & Puchades-Carrasco, L. (2019). Metabolomics contributions to the discovery of prostate cancer biomarkers. Metabolites, 9(3), 48.
Giskeødegård, G. F., Bertilsson, H., Selnæs, K. M., Wright, A. J., Bathen, T. F., Viset, T., et al. (2013). Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS ONE, 8(4), e62375.
Ho, S. M., Tang, W. Y., Belmonte de Frausto, J., & Prins, G. S. (2006). Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Research, 66(11), 5624–5632.
Hoeferlin, L. A., Wijesinghe, D. S., & Chalfant, C. E. (2013). The role of ceramide-1-phosphate in biological functions. Handbook of Experimental Pharmacology, 215, 153–166.
Hunter, W. G., Kelly, J. P., McGarrah, R. W., Khouri, M. G., Craig, D., Haynes, C., et al. (2016). Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: Evidence for shared metabolic impairments in clinical heart failure. Journal of the American Heart Assocciation, 5(8), e003190.
Jain, M., Nilsson, R., Sharma, S., Madhusudhan, N., Kitami, T., Souza, A. L., et al. (2012). Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science, 336(6084), 1040.
Jones, L. L., McDonald, D. A., & Borum, P. R. (2010). Acylcarnitines: Role in brain. Progress in Lipid Research, 49(1), 61–75.
Johnson, W. E., Li, C., & Rabinovic, A. (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8(1), 118–127.
Kdadra, M., Höckner, S., Leung, H., Kremer, W., & Schiffer, E. (2019). Metabolomics biomarkers of prostate cancer: A systematic review. Diagnostics (Basel, Switzerland), 9(1), 21. https://doi.org/10.3390/diagnostics9010021.
Knowles, R. G., & Moncada, S. (1994). Nitric oxide synthases in mammals. Biochemical Journal, 298(Pt 2), 249–258.
Köhler, E. S., Sankaranarayanan, S., van Ginneken, C. J., van Dijk, P., Vermeulen, J. L., Ruijter, J. M., et al. (2008). The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes. BMC Developmental Biology, 8, 107.
Krieger, J. N., Lee, S. W., Jeon, J., Cheah, P. Y., Liong, M. L., & Riley, D. E. (2008). Epidemiology of prostatitis. International Journal of Antimicrobial Agents, 31(Suppl 1), S85–90. https://doi.org/10.1016/j.ijantimicag.2007.08.028.
Kühn, T., Floegel, A., Sookthai, D., Johnson, T., Rolle-Kampczyk, U., Otto, W., et al. (2016). Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Medicine, 14, 13.
Law, S. H., Chan, M. L., Marathe, G. K., Parveen, F., Chen, C. H., & Ke, L. Y. (2019). An updated review of lysophosphatidylcholine metabolism in human diseases. International Journal of Molecular Sciences, 20(5), 1149.
Liepinsh, E., Makrecka-Kuka, M., Volska, K., Kuka, J., Makarova, E., Antone, U., et al. (2016). Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. The Biochemical Journal, 473(9), 1191–1202.
Mallah, K., Quanico, J., Raffo-Romero, A., Cardon, T., Aboulouard, S., Devos, D., et al. (2019). Matrix-assisted laser desorption/ionization-mass spectrometry imaging of lipids in experimental model of traumatic brain injury detecting acylcarnitines as injury related markers. Analytical Chemistry, 91(18), 11879–11887.
McCorquodale, D. J., & Mueller, G. C. (1958). Effect of estradiol on the level of amino acid-activating enzymes in the rat uterus. The Journal of Biological Chemistry, 232(1), 31–42.
McGill, M. R., Li, F., Sharpe, M. R., Williams, C. D., Curry, S. C., Ma, X., et al. (2014). Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Archives of Toxicology, 88(2), 391–401.
McNeill, A. M., Zhang, C., Stanczyk, F. Z., Duckles, S. P., & Krause, D. N. (2002). Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: Effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke, 33(6), 1685–1691.
Nakamura, N., Davis, K., Yan, J., Sloper, D. T., & Chen, T. (2020). Increased estrogen levels induced altered microRNA expression in prostate and plasma of rats dosed with sex hormones. Andrology. https://doi.org/10.1111/andr.12780.
National Research Council. (2011). Guide for the care and use of laboratory animals. Washington, DC: National Academy Press.
Nickel, J. C. (2011). Prostatitis. Canadian Urological Association Journal, 5(5), 306–315. https://doi.org/10.5489/cuaj.11211.
Nickel, J. C. (2012). Prostatitis and related conditions, orchitis, and epididymitis. In A. J. Wein, L. R. Kavoussi, A. C. Novick, A. W. Partin, & C. A. Peters (Eds.), Campbell-Walsh urology (pp. 327–356). Philadelphia: Saunders.
Payne, S. G., Milstien, S., & Spiegel, S. (2002). Sphingosine-1-phosphate: Dual messenger functions. FEBS Letters, 531(1), 54–57.
Perletti, G., Monti, E., Magri, V., Cai, T., Cleves, A., Trinchieri, A., et al. (2017). The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica, 89(4), 259–265. https://doi.org/10.4081/aiua.2017.4.259.
R Core Team. (2019). R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing.
Ren, S., Shao, Y., Zhao, X., Hong, C. S., Wang, F., Lu, X., et al. (2016). Integration of metabolomics and transcriptomics reveals major metabolic pathways and potential biomarker involved in prostate cancer. Molecular & Cellular Proteomics, 15(1), 154–163.
Richard, G., Batstone, D., & Doble, A. (2003). Chronic prostatitis. Current Opinion in Urology, 13(1), 23–29.
Russell, P. J., & Voeks, D. J. (2003). Animal models of prostate cancer. Methods in Molecular Medicine, 81, 89–112.
Rutkowsky, J. M., Knotts, T. A., Ono-Moore, K. D., McCoin, C. S., Huang, S., Schneider, D., et al. (2014). Acylcarnitines activate proinflammatory signaling pathways. American Journal of Physiology: Endocrinology and Metabolism, 306(12), E1378–E1387.
Schnackenberg, L. K., Pence, L., Vijay, V., Moland, C. L., George, N., Cao, Z., et al. (2016). Early metabolomics changes in heart and plasma during chronic doxorubicin treatment in B6C3F1 mice. Journal of Applied Toxicology, 36(11), 1486–1495.
Schooneman, M. G., Vaz, F. M., Houten, S. M., & Soeters, M. R. (2013). Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes, 62(1), 1–8.
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G., & De Marzo, A. M. (2018). The inflammatory microenvironment and microbiome in prostate cancer development. Nature Reviews Urology, 15(1), 11–24. https://doi.org/10.1038/nrurol.2017.167.
Slotte, J. P. (2013). Biological functions of sphingomyelins. Progress in Lipid Research, 52(4), 424–437.
Sorvina, A., Bader, C. A., Caporale, C., Carter, E. A., Johnson, I. R. D., Parkinson-Lawrence, E. J., et al. (2018). Lipid profiles of prostate cancer cells. Oncotarget, 9(85), 35541–35552.
Tong, Y.-C. (2011). The role of cholesterol in prostatic diseases. Urological Science, 22(3), 97–102.
Tracey, T. J., Steyn, F. J., Wolvetang, E. J., & Ngo, S. T. (2018). Neuronal lipid metabolism: Multiple pathways driving functional outcomes in health and disease. Frontiers in Molecular Neuroscience, 11, 10.
Treede, I., Braun, A., Sparla, R., Kühnel, M., Giese, T., Turner, J. R., et al. (2007). Anti-inflammatory effects of phosphatidylcholine. The Journal of Biological Chemistry, 282(37), 27155–27164.
van der Veen, J. N., Kennelly, J. P., Wan, S., Vance, J. E., Vance, D. E., & Jacobs, R. L. (2017). The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochimica et Biophysica Acta: Biomembranes, 1859(9 Pt B), 1558–1572.
Voelkel-Johnson, C., Norris, J. S., & White-Gilbertson, S. (2018). Interdiction of sphingolipid metabolism revisited: Focus on prostate cancer. Advantage of Cancer Research, 140, 265–293. https://doi.org/10.1016/bs.acr.2018.04.014.
Wang, W., Wu, Z., Dai, Z., Yang, Y., Wang, J., & Wu, G. (2013). Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids, 45(3), 463–477. https://doi.org/10.1007/s00726-013-1493-1.
Young, D. L. (1971). Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. Journal of Lipid Research, 12(5), 590–595.
Zhang, L., Wang, Y., Qin, Z., Gao, X., Xing, Q., Li, R., et al. (2020). Correlation between prostatitis, benign prostatic hyperplasia and prostate cancer: A systematic review and meta-analysis. Journal of Cancer, 11(1), 177–189. https://doi.org/10.7150/jca.37235.
Zhou, X., Mao, J., Ai, J., Deng, Y., Roth, M. R., Pound, C., et al. (2012). Identification of plasma lipid biomarkers for prostate cancer by lipidomics and bioinformatics. PLoS ONE, 7(11), e48889.
Zhu, Y. S. (2005). Molecular basis of steroid action in the prostate. Cellscience, 1(4), 27–55.
Acknowledgements
The authors thank Drs. Laura Schnackenberg and Pierre Alusta for their suggestions on the manuscript, and Dr. John K. Leighton (CDER) for his suggestions on this project proposal. The author (N.N.) thanks Mr. Ralph Patton and Ms. Kristie Voris, TPA, for plasma preparation, Ms. Sara Lewis for manuscript editing, and Ms. Patricia Shores and Ms. Kathy Carroll for preparing the cage cards.
Funding
This study was funded by NCTR/FDA (E0759701).
Author information
Authors and Affiliations
Contributions
NN conceived of the presented idea and experimental design. NN wrote the manuscript and finalized the manuscript, incorporating all of the co-authors’ comments. LMP performed the metabolomic analysis, analyzed data, and assisted with manuscript preparation. ZC performed the statistical analysis and provided Figures. RDB suggested the appropriate metabolomics analysis method. LMP, ZC, and RDB provided suggestions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All applicable guidelines set by the National Research Council’s “Guide for the Care and Use of Laboratory Animals” and the NCTR Institutional Animal Care and Use Committee were followed.
Informed consent
This study used animals and didn’t use any samples from humans. This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither this manuscript nor this study violates any of those policies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
The views expressed are those of the authors and do not represent the views of the U.S. Food and Drug Administration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakamura, N., Pence, L.M., Cao, Z. et al. Distinct lipid signatures are identified in the plasma of rats with chronic inflammation induced by estradiol benzoate and sex hormones. Metabolomics 16, 95 (2020). https://doi.org/10.1007/s11306-020-01715-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-01715-w