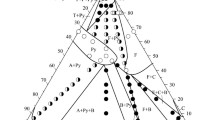
Phase equilibria and structural transformations in the ternary ZrO2–La2O3–Sm2O3 system at 1100°C were studied by X-ray diffraction over the entire composition range. Fields of solid solutions based on the hexagonal (A) modification of La2O3, cubic (F) modification with fluorite-type structure and tetragonal (T) and monoclinic (M) modifications of ZrO2, monoclinic (B) modification of Sm2O3, and an ordered intermediate phase with pyrochlore-type structure, Ln2Zr2O7 (Py), were established to exist in the system. The boundaries of phase fields and lattice parameters of the phases were determined. In the zirconia-rich corner, solid solutions based on the tetragonal modification of ZrO2 are formed. The solubility of La2O3 in T-ZrO2 is low and amounts to ~0.5 mol.%, which is evidenced by X-ray diffraction and microstructural analyses. The solid solutions based on the tetragonal modification of ZrO2 cannot be quenched from high temperatures in the cooling conditions. The X-ray diffraction patterns recorded at room temperature show peaks of the M-ZrO2 monoclinic phase. The ordered pyrochlore-type phase, Ln2Zr2O7 (Py), is in equilibrium with all phases that exist in the ternary ZrO2–La2O3–Sm2O3 system at 1100°C and forms substitutional solid solutions with phases of the binary systems. In the system, an infinite series of solid solutions form from the Ln2Zr2O7 (Py) phase. The isothermal section of the ZrO2–La2O3–Sm2O3 phase diagram at 1100°C contains three three-phase regions (T + M + Py, T + F + Py, A + B + Py) and eight two-phase regions (A + B, A + Py, B + Py, F + Py, F + T, T + M, T + Py, Py + M). New phases were not found to form at 1100°C.


Similar content being viewed by others
References
H.S. Zhang, Xu Qiang, F.C. Wang, and L. Liu, “Thermal conductivity of (La0.5Sm0.5)2(Zr0.9Ce0.1)2O7 ceramic for thermal barrier coatings,” Key Eng. Mater., 368–372, 1331–1333 (2008).
I.V. Mazilin, L.Kh. Baldaev, and D.V. Drobot, “Thermal and heat properties of lanthanum zirconate thermal barrier materials,” Perspekt. Mater., No. 7, 21–30 (2013).
G. Erdogan, F. Ustel, K. Bobzin, M. Öte, T.F. Linke, and L. Zhao, “Influence of long time post annealing on thermal stability and thermophysical properties of plasma sprayed La2Zr2O7 coatings,” J. Alloys Compd., 695, 2549–2555 (2017).
H. Zhang, K. Sun, Q. Xu, F. Wang, and L. Liu, “Thermal conductivity of (Sm1–xLax)Zr2O7 (x = 0, 0.25, 0.5, 0.75 and 1) oxides for advanced thermal barrier coatings,” J. Rare Earths, 27, 222–226 (2009).
A. Rouanet, “Contribution to the study of zirconia–lanthanide oxide systems near the melting point: Theses,” Rev. Int. Hautes Temper. Refract., 8, No. 2, 161–180 (1971).
E.R. Andrievskaya and L.M. Lopato, “Approximating the liquidus surface of the ZrO2–Y2O3–La2O3 phase equilibrium diagram with reduced polynomial,” Powder Metall. Met. Ceram., 39, No. 9–10, 445–450 (2000).
B. Bastide, P. Odier, and J.P. Coutures, “Phase equilibrium and martensitic transformation in lanthanadoped zirconia,” J. Am. Ceram. Soc., 71, No. 6, 449–453 (1988).
S.I. Schneider and R.S. Roth, “Phase equilibria in systems involving the rare-earth oxides. Part II. Solid state reactions in trivalent rare-earth oxide systems,” J. Res. Nat. Bur. Stand. A Phys. Chem., 64A, No. 4, 317–322 (1960).
M. Zinkevich, “Thermodynamics of rare earth sesquioxides,” Prog. Mater. Sci., 52, 597–647 (2007).
J. Coutures, A. Rouanet, R. Verges, and M. Foex, “High-temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. I. Phase diagrams (1400°C < T < T Liquide),” J. Solid State Chem., 17, No. 1–2, 171–182 (1976).
J. Coutures, F. Sibieude, and M. Foex, “High-temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. II. Influence of quenching on the nature of phases obtained at ambient temperature,” J. Solid State Chem., 17, No. 4, 377–384 (1976).
S.A. Toropov, Phase Diagrams of Refractory Oxide Systems [in Russian], Nauka, Leningrad (1987), p. 448.
Yumin Zhang, Thermodynamic Properties of Rare Earth Sesquioxides, Supervisor: Prof. In-ho Jung, McGill University, Montreal, QC, Canada (2016).
E.R. Andrievskaya, O.A. Kornienko, and Zh.D. Bogatyreva, “Phase relations in the La2O3–Sm2O3 system at 1500 °C,” Sovr. Probl. Fiz. Materialoved., No. 25, 15–28 (2016).
O.A. Kornienko, “Interaction of lanthanum and samarium oxides at 1250°C,” Ukr. Khim. Zh., 84, No. 3, 28–33 (2018).
Chong Wang, Matsvei Zinkevich, and Fritz Aldinger, “Experimental investigation and thermodynamic modeling of the ZrO2–SmO1.5 system,” J. Am. Ceram. Soc., 90, No. 7, 2210–2219 (2007).
V.B. Glushkova and L.V. Sazonova, “Effect of rare earth oxide additions on the polymorphism of zirconium dioxide,” in: Chemistry of High-Temperature Materials [in Russian], Nauka, Leningrad (1967), pp. 83–90.
M. Perez and Y. Jorba, “Contribution to the study of zirconia–rare earth oxide systems,” Ann Chim., 7, No. 7–8, 479–511 (1962).
E.I. Zoz, E.N. Fomichev, A.A. Kalashnik, and G.G. Eliseeva, “On the structure and properties of REM zirconates and hafnates,” 27, No. 1, 95–99 (1982).
E.R. Andrievskaya and L.M. Lopato, “Influence of composition on the T → M transformation in the systems ZrO2–Ln2O3 (Ln = La, Nd, Sm, Eu),” J. Mater. Sci., 36, No. 10, 2591–2596 (1995).
E.R. Andrievskaya, O.A. Kornienko, A.V. Samelyuk, V.S. Gorodov, K.A. Cherkasova, and V.O. Zgurovets, “Interaction of samarium oxide with zirconium oxide at 1500°C,” Sovr. Probl. Fiz. Materialoved., Issue 17, 16–24 (2008).
E.R. Andrievskaya, Phase Equilibria in the Hafnium, Zirconium, and Yttrium Oxides with Rare Earth Oxides [in Russian], Naukova Dumka, Kyiv (2010), p. 470.
E.R. Andrievskaya, O.A. Kornienko, V.S. Gorodov, K.A. Cherkasova, and I.S. Subota, “Interaction of cerium oxide with zirconium and lanthanum oxides at 1100°C,” Sb. Nauch. Tr. OAO UkrNII Ogneup., No. 108, 85–97 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
E. R. Andrievskaya is deceased
Translated from Poroshkova Metallurgiya, Vol. 59, Nos. 3–4 (532), pp. 138–148, 2020.
Rights and permissions
About this article
Cite this article
Korniienko, O.A., Bykov, A.I. & Andrievskaya, E.R. Phase Equilibria in the ZrO2–La2O3–Sm2O3 System at 1100°C. Powder Metall Met Ceram 59, 224–231 (2020). https://doi.org/10.1007/s11106-020-00154-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-020-00154-5