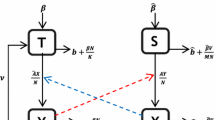
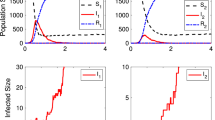
Abstract
Thresholds for disease extinction provide essential information for the prevention and control of diseases. In this paper, a stochastic epidemic model, a continuous-time Markov chain, for the transmission dynamics of West Nile virus in birds is developed based on the assumptions of its analogous deterministic model. The branching process is applied to derive the extinction threshold for the stochastic model and conditions for disease extinction or persistence. The probability of disease extinction computed from the branching process is shown to be in good agreement with the probability approximated from numerical simulations. The disease dynamics of both models are compared to ascertain the effect of demographic stochasticity on West Nile virus dynamics. Analytical and numerical results show differences in model predictions and asymptotic dynamics between stochastic and deterministic models that are crucial for the prevention of disease outbreaks. It is found that there is a high probability of disease extinction if the disease emerges from exposed mosquitoes unlike if it emerges from infectious mosquitoes and birds. Finite-time to disease extinction is estimated using sample paths and it is shown that the epidemic duration is shortest if the disease is introduced by exposed mosquitoes.




Similar content being viewed by others
References
Allen LJS (2008) An introduction to stochastic epidemic models. In: Brauer F, van den Driessche P, Wu J (eds) Mathematical epidemiology. Springer, Berlin, pp 77–128
Allen LJS (2010) An introduction to stochastic processes with applications to biology, 2nd edn. Chapman and Hall/CRC Press, Boca Raton
Allen LJS (2015) Stochastic population and epidemic models: persistence and extinction. Mathematical Biosciences Institute lecture series, stochastics in biological systems, vol 1.3. Springer International Publishing, Berlin
Allen LJS (2017) A primer on stochastic epidemic models: formulation, numerical simulation, and analysis. Infect Dis Modell 2:128–142
Allen LJS, Burgin AM (2000) Comparison of deterministic and stochastic SIS and SIR models in discrete time. Math Biosci 163:1–34
Allen LJS, Lahodny GE Jr (2012) Extinction thresholds in deterministic and stochastic epidemic models. J Biol Dyn 6:590–611
Allen LJS, van den Driessche P (2013) Relations between deterministic and stochastic thresholds for disease extinction in continuous- and discrete-time infectious disease models. Math Biosci 243:99–108
Anderson RM, May RM (1991) Infectious diseases of humans: dynamics and control, 2nd edn. Oxford University Press, London
Athreya KB, Ney PE (1972) Branching process. Springer, New York
Bartlett MS (1960) Stochastic population models. Methuen, London
Bartlett MS (1964) The relevance of stochastic models for large-scale epidemiological phenomena. Appl Stat 13:2–8. https://doi.org/10.2307/2985217
Bergsman LD, Hyman JM, Manore CA (2016) A mathematical model for the spread of West Nile Virus in migratory and resident birds. Math Biosci Eng. https://doi.org/10.3934/mbe.2015009
Botkin DB, Miller RS (1974) Mortality rates and survival of birds. Am Nat 108:181–192
Bowman C, Gumel A, van den Driessche P, Wu J, Zhu H (2005) A mathematical model for assessing control strategies against West Nile virus. Bull Math Biol 67:1107–1133
Bunning ML, Bowen RA, Cropp B, Sullivan K, Davis B, Komar N, Godsey M, Baker D, Jetter D, Holmes D et al (2001) Experimental infection of horses with West Nile virus and their potential to infect mosquitoes and serve as amplifying hosts. Ann N Y Acad Sci 951:338–339
CDC (2012) Statistics, surveillance, and control archive. http://www.cdc.gov/ncidod/dvbid/westnile/surv&control_archive.htm
Chatterjee S, Pal S, Chattopadhyay J (2008) Role of migratory birds under environmental fluctuation: a mathematical study. J Biol Syst 16:81–106
Chen J, Huang J, Beier JC, Cantrell RS, Cosner C, Fuller DO, Zhang G, Ruan S (2016) Modeling and control of local outbreaks of West Nile Virus in the United States. Discrete Continuous Dyn Syst Ser B 21(8):2423–2449
Chevalier V, Annelise T, Benoit D (2014) Predictive modeling of West Nile virus transmission risk in the Mediterranean basin: how far from landing? Int J Environ Res Public Health 11:67–90
Cruz-Pacheco G, Esteva L, Montao-Hirose J, Vargas C (2005) Modelling the dynamics of West Nile virus. Bull Math Biol 67:1157–1172
Diekmann O, Heesterbeek JA, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382
Ditlevsen S, Adeline S (2013) Introduction to stochastic models in Biology. In: Bachar M et al (eds) Stochastic biomathematical models, lecture notes in mathematics, vol 2058. Springer, Berlin. https://doi.org/10.1007/978-3-642-32157-3_1
Eidson M, Kramer L, Stone W, Hagiwara Y, Schmit K, New York State West Nile virus Avian Surveillance Team (2001) Dead bird surveillance as an early warning system for West Nile virus. Emerg Infect Dis 7:631–635
Harris TE (1963) The theory of branching processes. Springer, Berlin
Hayes EB, Gubler DJ (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57:181–194
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42:599–653
Kirupaharan N, Allen LJS (2004) Coexistence of multiple pathogen strains in stochastic epidemic models with density-dependent mortality. Bull Math Biol 66:841–864
Komar N (2003) West Nile virus: epidemiology and ecology in North America. Adv Virus Res 61:185–234
Lahodny GE Jr, Allen LJS (2013) Probability of a disease outbreak in stochastic multipatch epidemic models. Bull Math Biol. https://doi.org/10.1007/s11538-013-9848-z
Lahodny GE Jr, Gautam R, Ivanek R (2015) Estimating the probability of an extinction or major outbreak for an environmentally transmitted infectious disease. J Biol Dyn 9:128–155
Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M et al (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337
Laperriere V, Brugger K, Rubel F (2011) Simulation of the seasonal cycles of bird, equine and human West Nile virus cases. Prev Vet Med 98:99–110
Lloyd AL, Zhang J, Root AM (2007) Stochasticity and heterogeneity in host-vector models. J R Soc Interface 4:851–863
Mackenzie J, Gubler D, Petersen L (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10:S98–S109
Maliyoni M, Chirove F, Gaff HD, Govinder KS (2017) A stochastic tick-borne disease model: exploring the probability of pathogen persistence. Bull Math Biol 79:1999–2021
Maliyoni M, Chirove F, Gaff HD, Govinder KS (2019) A stochastic epidemic model for the dynamics of two pathogens in a single tick population. Theor Popul Biol 127:75–90
Marfin AA, Gubler DJ (2001) West Nile encephalitis: an emerging disease in the United States. Clin Infect Dis 33:1712–1719
McCormack RK, Allen LJS (2005) Disease emergence in deterministic and stochastic models for host and pathogen. Appl Math Comput 168:1281–1305
Mollison D (1991) Dependence of epidemic and population velocities on basic parameters. Math Biosci 107:255–287
Mpeshe SC, Haario H, Tchuenche JM (2011) A mathematical model of Rift Valley fever with human host. Acta Biotheor 59:231–250
Mwamtobe PM, Simelane SM, Abelman S, Tchuenche JM (2017) Mathematical analysis of a lymphatic filariasis model with quarantine and treatment. BMC Public Health 17:265
Petersen LR, Roehrig JT (2001) West Nile virus: a reemerging global pathogen. Emerg Infect Dis 7:611–614
Qiu Z (2011) Dynamics of an epidemic model with host migration. Appl Math Comput 218:4614–4625
Rossi S, Ross T, Evans J (2010) West Nile virus. Clin Lab Med 30:47–65
Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, Niedrig M, Papa A, Pierro A, Rossini G et al (2013) West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect 19:699–704
Sani A, Kroese DP, Pollett PK (2006) Stochastic models for the spread of HIP in a mobile heterosexual population. Math Biosci. https://doi.org/10.1016/j.mbs.2006.09.024
Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML (2001) Vector competence of selected North American culex and coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis 7(6):1018–22
Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA (2008) Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc R Soc Lond B Biol Sci 279:925–933
Steele KE, Linn MJ, Schoepp RJ, Komar N, Geisbert TW, Manduca RM, Calle PR, Raphael BL, Clippinger TL, Larsen T, Smith J, Lanciotti RS, Panella NA, Mc Namara TS (2000) Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City. Vet Pathol 37:208–224
Thomas DM, Urena B (2001) A model describing the evolution of West Nile-like encephalitis in New York City. Math Comput Model 34:771–781
Unnasch R, Sprenger T, Katholi C, Cupp E, Hill G, Unnasch T (2006) A dynamic transmission model of eastern equine encephalitis virus. Ecol Modell 192:425–440
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Wan H, Zhu H (2010) The backward bifurcation in compartmental models for West Nile virus. Math Biosci 227:20–28
Whittle P (1955) The outcome of a stochastic epidemic: a note on Bailey’s paper. Biometrika 42:116–122
Wonham MJ, Lewis MA (2008) A comparative analysis of models for West Nile virus. In: Brauer F, van den Driessche P, Wu J (eds) Mathematical epidemiology. Lecture notes in mathematics, vol 1945. Springer, Berlin, pp 365–390
Wonham MJ, de Camino-Beck T, Lewis MA (2004) An epidemiological model for West Nile virus: invasion analysis and control applications. Proc R Soc Lond B Biol Sci 271:501–507
Acknowledgements
I am grateful to the University of Malawi, Chancellor College for sponsoring my doctoral studies at the University of KwaZulu-Natal (UKZN) in South Africa. I extend my heartfelt gratitude to Dr. Faraimunashe Chirove (University of Johannesburg, South Africa) Professor Keshlan S. Govinder (UKZN), and Professor Holly D. Gaff (Old Dominion University, USA) for mentoring me and introducing me to stochastic epidemic modelling when I was their PhD student from November 2015 to November 2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maliyoni, M. Probability of Disease Extinction or Outbreak in a Stochastic Epidemic Model for West Nile Virus Dynamics in Birds. Acta Biotheor 69, 91–116 (2021). https://doi.org/10.1007/s10441-020-09391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-020-09391-y