Absract
Purpose
To demonstrate that oral drug absorption is terminated in finite time. To develop models based on biopharmaceutical/physiological and finite absorption time concepts.
Methods
The models are based on i) the passive drug diffusion mechanism under the sink conditions principle ii) the rate limiting role of the drug’s properties solubility and permeability and iii) the relevant restrictions associated with the gastrointestinal transit times of drug in the stomach, the small intestines and the colon. Two input functions of constant rate are considered for the absorption of drug from i) the stomach/small intestines with an upper limit of 5 h and ii) the colon with an upper limit of 30 h. Branched differential equations were written for the time course of drug in the body.
Results
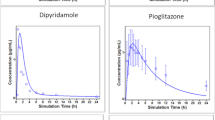
Simulations were performed using different scenarios, assuming a variety of drug properties and limited or non-existent absorption from the colon. Literature oral data of cephradine, ibuprofen, flurbiprofen and itraconazole were analyzed. For all drugs examined, nice fittings of the branched differential equations to the experimental data were observed.
Conclusions
For all drugs the absorption process was terminated in the small intestine. The meaning of partial AUCs, Cmax, tmax are questioned. Applications of these models to IVIVC are anticipated.






Similar content being viewed by others
Change history
29 September 2020
A Correction to this paper has been published: https://doi.org/10.1007/s11095-020-02935-4
Abbreviations
- BCS:
-
Biopharmaceutic classification system
- BDDCS:
-
Biopharmaceutic drug disposition classification System
- GI:
-
Gastrointestinal
- IVIVC:
-
In vitro in vivo correlations
- PBFTPK:
-
Physiologically based finite time pharmacokinetic
- PBPK:
-
Physiologically based pharmacokinetic
References
Amidon GL, Lennernäs H, Shah VP, Crison JR. “A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability”. In: Pharm Res 12.3 (1995), pp. 413–20. [Journal Article].
Wu C, Benet L. “Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system”. In: Pharm Res 22.1 (2005), pp. 11–23. [Journal Article].
Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Guidance for Industry U.S. Department of Health and Human Services. 2017. [Scientific Guideline].
European Medicines Agency. Committee for medicinal products for human use (CHMP) Guideline on the investigation of bioequivalence. London, Jan. 2017.[Scientific Guideline].
Charalabidis A, Sfouni M, Bergström C, Macheras P. “BCS and BDDCS: Beyond guidelines”. In: International Journal of Pharmaceutics (2019), 566:264–281. [Journal Article].
Macheras P, Karalis V, Valsami G. “Keeping a critical eye on the science and the regulation of oral drug absorption: a review”. Journal of Pharmaceutical Sciences102, 3018–3036 (2013).[Journal Article].
Yu LX and Amidon GL (1999) “A compartmental absorption and transit model for estimating oral drug absorption”. In: Int J Pharm 186:119-125. [journal article].
Lin L, Wong H. “Predicting Oral Drug Absorption: Mini Review on Physiologically-Based Pharmacokinetic Models”. In: Pharmaceutics. 2017;9(4):41. [Journal Article].
Chung J, Kesisoglou F. “Physiologically Based Oral Absorption Modelling to Study Gut-Level Drug Interactions”. In: J Pharm Sci.2018;107(1):18–23. [Journal Article].
Stillhart C, Pepin X, Tistaert C, et al. “PBPK Absorption Modeling: Establishing the In Vitro-In Vivo Link-Industry Perspective”. In: AAPS J.2019;21(2):19. [Journal Article].
Rinaki E, Dokoumetzidis A, Valsami G, Macheras P. “Identification of biowaivers among class II drugs: theoretical justification and practical examples”. In : Pharmaceutical Research.21, 1567–1572 (2004). [Journal Article].
Macheras P, Karalis V. “A non-binary biopharmaceutical classification of drugs : the ΑΒΓsystem”. In: International Journal of Pharmaceutics 464 (2014), pp. 85–90. [Journal Article].
Macheras P. “On an Unphysical Hypothesis of Bateman Equation and its Implications for Pharmacokinetics”. In: Pharmaceutical research 36 (July 2019), p. 94. [Journal Article].
Dost F. H. “Der Blutspiegel. Kinetik der Konzentrationsverläufe in der Kreislaufflüssigkeit”. In: Leipzig (Sept. 1953). [Journal Article].
H. Bateman. “Solution of a system of differential equations occurring in the theory of radioactive transformations”. In: Proc. Cambridge Philos. Soc. 15 (1910), pp. 423–427. [Journal Article].
Garrett Edward R. “The Bateman function revisited: A critical reevaluation of the quantitative expressions to characterize concentrations in the one compartment body model as a function of time with first-order invasion and first-order elimination”. In: Journal of Pharmacokinetics and Biopharmaceutics, 22.2 (Apr. 1994), pp. 103–128. [Journal Article].
Abuhelwa A, Foster D, Upton R. “A Quantitative Review and Meta-models of the Variability and Factors Affecting Oral Drug Absorption-Part II: Gastrointestinal Transit Time”. In: AAPS J 18.5 (Sept. 2016), pp. 1322–1333. DOI: https://doi.org/10.1208/s12248- 016- 9953- 7. [Journal Article].
Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. Wolters Kluwer Health/Lippincott William Wilkins, 2011. [Book].
Niazi S. Textbook of biopharmaceutics and clinical pharmacokinetics.Chapter 7. 292 Madison Ave., New York: Appleton-Century-Crofts, 1979. [Book].
Chen ML. “An Alternative Approach for Assessment of Rate of Absorption in Bioequivalence Studies”. In: Pharmaceutical Research 9.11 (Apr. 1992). [Journal Article].
Szpunar GJ, Albert KS, Bole GG, Dreyfus JN, Lockwood GF, Wagner JG. “Pharmacokinetics of flurbiprofen in man I. Area/dose relationships”. In: Biopharmaceutics Drug Disposition 8 (1987), pp. 273–283. [Journal Article].
Hardin TC, Graybill JR, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JG. “Pharmacokinetics of Itraconazole following Oral Administration to Normal Volunteers”. In: Antimicrobial Agents and Chemotherapy 32.9 (Dec. 1988), pp. 1310–1313. [Journal Article].
Arfken GB, Weber HJ, Harris. Mathematical Methods for Physicists. Seventh Edition. 225 Wyman Street Waltham MA 02451 USA: Elsevier, 2013. [Book].
Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes 3rd Edition: The Art of Scientific Computing. Cambridge University Press, 2007. [Book].
Vertzoni M, Augustijns P, Grimm M, Koziolek M, Lemmens G, Parrott N, Pentafragka C, Reppas C, Rubbens J, Van Den Αbeele J, Vanuytsel T, Weitschies W, Wilson CG. “Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review”. In: Eur J Pharm Sci 134 (June 2019), pp. 153–175. DOI: https://doi.org/10.1016/j.ejps.2019.04.013. [Journal Article].
Kararli T, Searle GD. “Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals”. In: Biopharmaceutics and Drug Disposition 16 (1995), pp. 351–380. DOI: https://doi.org/10.1002/bdd.2510160502. [Journal Article].
Iranpour P, Lall C, Houshyar R, Helmy M, Yang A, Choi JI, Ward G, Goodwin SC. “Altered Doppler flow patterns in cirrhosis patients: an overview”. In: Ultrasonography 35.1 (Jan. 2016), pp. 3–12. DOI: https://doi.org/10.14366/usg.15020. [Journal Article].
Digenis GA, Sandefer EP, Parr AF, Beihn R, McClain C, Scheinthal BM, Ghebre-Sellassie I, Iyer U, Nesbitt RU, Randinitis E. “Gastrointestinal behavior of orally administered radiolabeled erythromycin pellets in man as determined by gamma scintigraphy”. In: Journal of Clinical Pharmacology 30.7 (July 1990), pp. 621–31. [Journal Article].
Dokoumetzidis A, Iliadis A, Macheras P. “An alternative method for the estimation of the terminal slope when a few data points are available”. In: Journal of Pharmaceutical Sciences 88 (Apr. 1999), pp. 557–560. [Journal Article].
Macheras P, Dokoumetzidis A. “On the use of partial AUC as an early exposure metric”. In: European Journal of Pharmaceutical Sciences 10 (2000), pp. 91–5. [Journal Article].
Macheras P, Symillides M, Reppas C. “The cut-off time point of the partial area method for assessment of rate of absorption in bioequivalence studies”. In: Pharmaceutical Research 11 (1994), pp. 831–834. [Journal Article].
Sanchez-Navarro M, Teixido M, Giralt E. “Jumping hurdles: peptides able to overcome biological barriers”. In: Acc Chem Res 50.8 (2017), pp. 1847–1854. DOI: 10 . 1021 / acs. accounts. 7b00204. [Journal Article].
Macheras P, Iliadis A, Melagraki G. A reaction limited in vivo dissolution model for the study of drug absorption: Towards a new paradigm for the biopharmaceutic classification of drugs. Eur J Pharm Sci. 2018;117:98–106.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Macheras, P., Chryssafidis, P. Revising Pharmacokinetics of Oral Drug Absorption: I Models Based on Biopharmaceutical/Physiological and Finite Absorption Time Concepts. Pharm Res 37, 187 (2020). https://doi.org/10.1007/s11095-020-02894-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02894-w