Abstract
Climate change may alter microscale-effective ecosystem properties such as atmospheric water vapour pressure, but consequences for plant growth are insufficiently understood. Within a northwest German heathland an open-top chamber experiment was established to analyse the effects of elevated vapour pressure deficit (eVPD) on growth responses of Calluna vulgaris considering both plant origin (Atlantic (AP), sub-Atlantic (SAP), sub-Continental (SCP)) and life-history stage (1-year vs. 10-year old plants). We hypothesised that the plants’ sensitivity to eVPD decreases (i) from AP to SCP and (ii) with progressing life-history stage. Elevated VPD caused a provenance-specific decrease of shoot increment whilst aboveground biomass productivity remained unaffected. AP and SAP responded with increasing belowground biomass δ13C signatures to eVPD, whereas δ13C values decreased for SCP. Moreover, eVPD increased and decreased belowground biomass δ13C signatures of 1- and 10-year old plants, respectively. These responses to eVPD were related to differences in morphological-chemical traits and the plants’ trait plasticity in response to eVPD. SCP showed the highest aboveground tissue mass density and significantly increased tissue C:N ratios under eVPD. One-year old plants had a tenfold higher shoot:root ratio than 10-year old plants, making young plants more sensitive to eVPD. Our findings demonstrate that the atmospheric water status affects the morphology and physiology of Calluna independent of the soil water status. The results have implications for the conservation of heathlands under climate change: (i) SCP may constitute an appropriate ecotype for assisted migration-approaches, and (ii) management needs to weigh different options for heathland rejuvenation.
Similar content being viewed by others
Introduction
Heathlands represent one of the oldest cultural landscapes in Europe, are home to a critical proportion of the biodiversity typical of open acidic habitats (Gimingham 1972) and, as such, are of high conservation value (NATURA 2000 Habitat Directive). Nutrient-poor (and foremost N-limited) lowland heaths are often dominated by the ericaceous dwarf shrub Calluna vulgaris (L.) Hull (henceforth referred to as Calluna). As a perennial plant, Calluna passes through various life-history stages—namely the pioneer, building, mature and degenerate stage—during which several morphological-chemical key traits can change (Watt 1947, 1955; Gimingham 1960). As a result, the sensitivity of Calluna to global change drivers such as climate change may vary with plant age.
The distribution range of Calluna-heathlands spans the area of W Europe (Gimingham 1972), the climate of which is characterised by a general increase in continentality from its north-western to south-eastern borders (Loidi et al. 2010). According to current climate projections, precipitation patterns will shift towards drier summers within this distribution range (IPCC 2018). For NW Germany, for example, which is home to one of the largest sub-Atlantic Calluna-heathlands, a decrease in summer precipitation of up to 10% is expected (May et al. 2016), accompanied by a temperature increase of up to 1.0–1.5 °C by the mid-twenty-first century (Wagner et al. 2013). Such shifts in climate would result in reduced soil water availability and even soil borne water limitation, particularly for edaphically dry sites which are typical of many Calluna-dominated lowland heaths. Several studies have demonstrated that growth and vitality of Calluna may be negatively affected with increasing water shortage (e.g. Peñuelas et al. 2004; Llorens et al. 2004; Albert et al. 2011, 2012).
Interestingly, a recent study found that drought sensitivity of Calluna decreases with progressing life-history stage (Meyer-Grünefeldt et al. 2015), attributable to age-related shifts in shoot to root ratios (henceforth referred to as shoot:root ratio) that co-determine a plant’s water uptake and transpiration rates (Weiner et al. 2004). Moreover, Meyer-Grünefeldt et al. (2016) found that 1-year old Calluna seedlings from eastern and southern European range margins are more resistant to drought in comparison to those from central European populations, likely a consequence of local adaptations to climatically controlled growth conditions (Rose et al. 2009).
While a reduction of precipitation rates can lead to lower soil water contents, increasing temperatures may cause a decrease of the near-surface relative humidity (Clausius-Clapeyron relationship; O’Gorman and Muller 2010; Collins et al. 2013), which in turn is associated with an increase in the water vapour pressure deficit (VPD) of the air. The leaf-to-air water vapour pressure difference is a key abiotic factor, since it drives a plant’s transpiration rate and its water status (Tibbitts 1979; Ford and Thorne 1974; Leuschner 2002; Lendzion and Leuschner 2009; Lihavainen et al. 2016). As a consequence, increasing temperatures can increase plant water deficits and lead to a reduction in biomass productivity, even under ample soil water supply. This has been shown for European beech (Fagus sylvatica) saplings and temperate forest herbs grown under elevated VPD (i.e. under drier climate, but ample soil water availability; henceforth referred to as eVPD; Lendzion and Leuschner 2008). In this way, increasing summer temperatures have the potential to affect a species’ distribution range via eVPD (Lendzion and Leuschner 2008).
To the best of our knowledge, to date only one study has examined the impact of VPD on Calluna as an understorey plant species in temperate forests (Gobin et al. 2015). The authors found that there was little or no regulation of transpiration in response to eVPD. However, the effects of eVPD on growth and performance of Calluna plants originating from different provenances or different life-history stages are unknown. It is, for example, conceivable that Calluna plants show ecotype- or life-history stage-specific responses to shifts in VPD. With such knowledge it would subsequently be possible to better understand and predict the species’ resistance or resilience to climatic shifts. This in turn would allow for an adaptation of management strategies to mitigate the impacts of climate change on Calluna-heathlands.
In the present study we quantified the effects of eVPD on growth of Calluna plants by means of an open-top chamber experiment carried out in a lowland heath in NW Germany. To test for provenance-effects, we analysed growth responses (biomass and allocation, specific shoot length, tissue δ13C signatures and tissue C:N ratios) of 1-year old plants raised from seeds collected from three different provenances (i.e. an Atlantic, a sub-Atlantic, and a sub-Continental provenance; henceforth referred to as AP, SAP, and SCP, respectively). To test for the effects of life-history stage, we compared growth responses of 1-year old and 10-year old plants (originating from the SAP) to eVPD. We expected that eVPD has a negative effect on growth of Calluna plants, reflected by decreasing biomass and increasing tissue δ13C signatures, but that growth responses were provenance- and life-history stage-specific. We hypothesised that (i) Calluna plants from AP and SAP are more sensitive to eVPD than plants from SCP (due to provenance-specific adaptations to local climates), and that (ii) Calluna plants from early life-history stages are more sensitive to eVPD than plants from later life-history stages (e.g. due to differences in morphological traits such as shoot:root ratios).
Material and methods
Experimental design
An in situ simulation of a drier heathland microclimate (i.e. with eVPD) was performed by means of an open-top chamber (OTC) system during the growing season in 2016 (May to October; following the experimental set-up of Lendzion and Leuschner (2009), which allows for an optimal maintenance of all other environmental variables). The system was established in a heathland area in Reinsehlen (53°9′ N, 9°48′ E), NW Germany, adjacent to the Lueneburg Heath nature reserve (e.g. Cordes et al. 1997). Calluna plants showed a well-established bush-like form and achieved a maximum height of ca. 30–40 cm, with an age-range of the oldest individuals between 10 and 18 years (inferred from annual ring counts at the stem basis of ten individuals). The OTCs used in the experiment measured 0.65 m in diameter and 0.60 m in height and were made of UV-transmissive plexiglass. OTCs were connected to two large radial fans through plastic pipes, which channelled the air into five OTCs per treatment, i.e. a control with ambient VPD and a treatment with eVPD. Two absorption air driers (Resuscorb, DST Seibu Giken, Sweden) were used to cause a reduction of the air humidity by 26% in the OTCs subjected to the eVPD treatment relative to those in the control (Table 1). A cross-flow heat exchanger (Duplexvent 15,000, Airflow Lufttechnik GmbH, Rheinbach, Germany) matched the air temperature in both streams to avoid air temperature differences between treatments. OTCs were assigned randomly to the treatments (i.e. control and eVPD; see Fig. 1, also for the number of replicates per treatment). Within the homogenous 10-year old Calluna stands embraced by each OTC, three subplots (each of which 10 cm × 10 cm in size) were established by removing the vegetation and organic layer from the prevailing podzol soil profile. The subplots were then re-filled with humus collected from the organic horizon of an adjacent podzol soil within the nature reserve Lueneburg Heath (soil ecological characteristics: pHH20: 4.1, cation exchange capacity: 4.8 mmolc 100 g−1, 0.08% soil N content). Between October and November 2015, Calluna seeds were collected from three different provenances (AP: Lygra Heath, W Norway, SAP: Lueneburg Heath, NW Germany, SCP: Oranienbaum Heath, E Germany; for climate characteristics of the three sites see Table 2). In January 2016, 1000 Calluna seeds per provenance were sown into a randomly chosen subplot of an OTC, so that all Calluna provenances were present in one OTC. The number of seeds sown per subplot accounted for the low germination rate known for Calluna (< 10%; Thomas and Davies 2002). Mean germination rates for AP, SAP and SCP were 0.6%, 0.4% and 0.5%, respectively, leading to a final plant density of 6, 4 and 5 plants per subplot.
Illustration of the experimental set-up and the installation of open-top chambers. Circles and rectangles symbolise open-top chambers and corresponding air-channel systems, respectively. Dark and light grey areas represent control (C) open-top chambers (n = 5) and open-top chambers with elevated vapour pressure deficit (eVPD; n = 5), respectively
All OTCs were continuously watered throughout the growing season, keeping the soil water content close to water holding capacity. During the experiment, air temperature (Tair, °C) and relative humidity (RHair, %) inside and outside of OTCs were recorded by means of capacitive air humidity sensors (HygroClip, Rotronic AG, Etlingen, Germany) every ten minutes at 0.5 m above ground level. Daily air VPD (in Pa; Eq. 1) was calculated as the difference between saturated (Psat; Eq. 2) and effective water vapour pressure of the air (Pair; Eq. 3; Gobin et al. 2015; Monteith and Unsworth 2008; WMO 2008).
Growing season (May to October 2016) means of VPD, relative humidity, and air temperature are presented in Table 1.
Sampling procedure and analyses
One-year old plants
To determine the annual shoot increment of 1-year old Calluna plants, the height of each individual per subplot and OTC was measured in October 2016 using a calliper gauge. The plants’ shoot:root ratio (as a trait describing aboveground/belowground biomass allocation patterns; Weiner 2004; Meyer-Grünefeldt et al. 2015) was quantified after harvesting each individual at the end of the experiment (October 2016). To this end, the plants’ above- and belowground biomass was separated, roots carefully washed until soil residues were removed as far as possible, biomass material dried at 40 °C for 48 h and subsequently weighed. As a further (provenance-specific) morphological characteristic we calculated the specific shoot length as the ratio of shoot increment to aboveground biomass dry weight, following the approaches of Fitter (1997) and Wilson et al. (1999). Specific shoot length represents a ratio of standard unit of acquisition (shoot length) to resource investment (mass) and provides a proxy for tissue mass density (e.g. the proportion of sclerenchymatic tissue; Wahl and Ryser 2000; Zhang et al. 2017; Guerra and Scremin-Dias 2018). In addition to biomass, we analysed aboveground and belowground tissue δ13C signatures and C:N ratios. Tissue δ13C signatures were used as a rough proxy for plant water deficits, since values are related to stomatal conductance and the ratio of intracellular to external CO2 concentration (Farquhar et al. 1982, 1989).
Biomass samples were dried, ground with a mixer mill (MM 400, Retsch, Haan, Germany), and re-dried at 40 °C for 48 h. C and N contents as well as tissue δ13C signatures were measured using a continuous flow element analyser-isotope mass spectrometer (vario EL cube, Elementar, Hanau, Germany, coupled to an Isoprime IRMS, Isoprime Ltd., Cheadle, Hulme, UK). Isotope signatures were presented in the delta (δ) notion in per mil (‰) as the relative deviation from an international standard (PeeDee Belemnite). The relative precision of repeated analyses of IAEA standards (IAEA-CH-3) was ± 0.1‰.
Ten-year old plants
The annual shoot increment of the 10-year old Calluna plants was quantified at the end of the growing season by measuring the length of the five longest current year’s shoots at the longest last year’s leading shoots of 20 randomly chosen plants per OTC (von Oheimb et al. 2010). To estimate the total plant biomass at the end of the experiment, aboveground biomass of all plants within each OTC was cut off directly over the soil surface, dried at 40 °C for 48 h, and weighed. Belowground biomass was determined by taking three randomly located root samples per OTC (using cylindrical soil insertion rings with 8 cm in diameter and 30 cm in length; Eijkelkamp Soil & Water, Giesbeek, Netherlands). Root samples were carefully washed to remove adhered soil material, dried at 40 °C for 48 h, and weighed. Analyses of tissue C:N ratios and δ13C signatures of aboveground and belowground biomass samples followed the procedure described for 1-year old Calluna plants (see above).
Statistical analyses
We applied linear mixed-effects models to analyse the effects of eVPD on morphological and physiological responses of Calluna. To test for provenance- and life-history stage-specific responses of Calluna to changes in eVPD, we fitted two separated models that included the fixed effects (i) ‘treatment’ (control vs. eVPD), ‘provenance’ (AP, SAP, and SCP) and the interaction treatment × provenance, and (ii) ‘treatment’, ‘plant age’ (1-year old plants vs. 10-year old plants) and the interaction treatment × plant age. ‘OTC’ was used as random effect in both models. Significance of predictors was assessed by likelihood ratio tests based on maximum likelihood (ML) estimation (Zuur et al. 2009). Prior to analysis, all response variables (except δ13C signatures) were log-transformed to meet model assumptions. Model assumptions were checked and confirmed according to Zuur et al. (2009).
For several response variables (above- and belowground biomass, shoot:root ratio, specific shoot length, belowground biomass C and N content, above- and belowground biomass C:N ratio, and belowground biomass δ13C signature), we excluded measurements with extreme positive or negative values (e.g. measurement errors; 4.8% of the data). Omitted values, for example, concerned damaged Calluna individuals (e.g. with broken or missing main shoots and therefore incorrect values for aboveground biomass or shoot:root ratios) and were considered outliers according to the definition of Crawley (2007). A post hoc test (Tukey’s HSD test) was performed for multiple between-provenances comparisons. All analyses were conducted in R version 3.6.2 (R Core Team 2019) using the packages nlme (Pinheiro et al. 2010), and multcomp (Hothorn et al. 2008).
Results
Effects of eVPD on provenances and comparison of provenances
Belowground biomass δ13C signatures responded differently to eVPD: Whereas values of AP and SAP increased, values of SCP decreased. This opposing response pattern was reflected by a marginally significant provenance × eVPD interaction effect (P = 0.078, Table 3). A similar but non-significant trend was found for δ13C signatures of the aboveground biomass (Fig. 2; Tables S1, S4). Further, eVPD caused a marginally significant decrease of the specific shoot length of Calluna plants (for AP and SAP; P = 0.064, Table 3; Fig. 2b). This response was related to a reduced shoot increment (particularly for AP, P < 0.1, Table S1), with concomitantly unaltered aboveground biomass productivity under eVPD (Tables 3, S1). In addition, eVPD significantly decreased aboveground biomass N contents (P = 0.043, Table 3; particularly for AP and SCP, Table S1), resulting in increased aboveground C:N ratios across provenances (e.g. aboveground biomass C:N ratios of SCP increased from 23.8 to 31.3; Table S1).
Provenance-related effects of elevated vapour pressure deficit (eVPD) on: a shoot:root ratio, b specific shoot length (mm/g), c aboveground tissue δ13C signatures (‰), d belowground tissue δ13C signatures (‰), e aboveground biomass C:N ratio, and f belowground biomass C:N ratio of 1-year old Calluna vulgaris. Grey symbols show observed treatment-(control (C) vs. eVPD) and provenance-specific (AP: Atlantic, SAP: sub-Atlantic, and SCP: sub-Continental) values, while black squares and error bars show observed means and standard errors (± 1SE), respectively. Letters indicate significant differences between provenances (Tukey’s HSD test; α = 0.05). Asterisks indicate a significant treatment effects within a specific provenance (P ≤ 0.05)
Independently of the effects of eVPD, we found several provenance-specific trait characteristics (Table S2). SCP showed the highest shoot increment, aboveground biomass productivity and shoot:root ratio (controls; Tables 3, S1).
Effects of eVPD on life-history stages and comparison of life-history stages
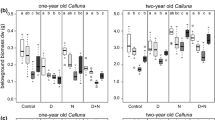
Belowground biomass δ13C values for 1-year old plants (trend) increased under eVPD, but decreased for 10-year old plants (Fig. 3c; P < 0.1, Table S1). This response was reflected by the results of the mixed model, according to which eVPD significantly affected belowground tissue δ13C signatures (P = 0.004, Table 3; Fig. 3c). In addition, aboveground biomass C:N ratios strongly increased under eVPD (P = 0.014, Table 3), particularly for 10-year old plants (Table S1; Fig. 3d) due to a reduction in aboveground biomass N contents (P = 0.026, Table 3). In addition, belowground biomass C contents showed an age-specific response to eVPD (age × eVPD interaction effect, P = 0.029, Table 3), with increasing and decreasing values for 10-year old and 1-year old plants, respectively. This response caused a concomitant shift in belowground biomass C:N ratios (age × eVPD interaction effect, P = 0.083, Table 3). Interestingly, shoot:root ratios increased for 10-year old plants, but decreased for 1-year old plants under eVPD, reflected by an age × eVPD interaction effect (P = 0.035, Tables 3, S1).
Age-related effects of elevated vapour pressure deficit (eVPD) on: a shoot:root ratio, b aboveground tissue δ13C signatures (‰), c belowground tissue δ13C signatures (‰), d aboveground biomass C:N ratio, and e belowground biomass C:N ratio of Calluna vulgaris. Grey and white dots show observed treatment- (control (C) vs. eVPD) and life-history stage-specific (1-, and 10-year old Calluna vulgaris from a sub-Atlantic provenance) values, while black squares and error bars show observed means and standard errors (± 1SE), respectively. Letters indicate significant differences between life-history stages (P ≤ 0.05). Asterisks indicate significant treatment effects within a specific life-history stage (P ≤ 0.05)
Independent of the effects of eVPD, we found highly significant differences in morphological and chemical variables between life-history stages (Table S3). Shoot:root ratios were about ten times higher for 1-year old compared to 1-year old plants (4.10 vs. 0.38 in controls), whereas 10-year old plants showed higher biomass C:N ratios, particularly for belowground tissue (82.9 vs. 53.1 in the control, Table S1).
Discussion
Provenance-specific sensitivity of Calluna to eVPD
For 1-year old Calluna plants, we found a provenance × eVPD interaction effect for belowground biomass δ13C signatures. This finding supports our first hypothesis that plants from different provenances show opposing responses to eVPD, with AP and SAP being more sensitive to eVPD than SCP. Increasing tissue δ13C signatures of AP and SAP plants indicate increasing stomata closure due to eVPD and hence decreasing 13C discrimination. In contrast, stomatal conductance of SCP plants (in tendency) remained unchanged or even increased under eVPD (Wong et al. 1979; Ball and Farquhar 1984). Obviously, plants from SCP did not adjust for eVPD (in terms of stomatal regulation), or even increased transpiration rates, presumably driven by the higher atmospheric demand and apparently unrestricted by the hydraulic conductivity of the plants’ root system (Sadok and Sinclair 2010; Ocheltree et al. 2014; Gobin et al. 2015).
We hypothesise that different responses of provenances are related to both provenance-specific (morphological or chemical) trait characteristics and plastic responses of traits to eVPD (trait plasticity sensu Weiner (2004)). On the one hand, SCP showed the lowest specific shoot length, indicating that these plants exhibit higher aboveground tissue mass densities than plants of AP and SAP. High tissue mass densities in turn are often related to a high proportion of sclerenchymatic tissue (Garnier and Laurent 1994; Van Arendonk and Poorter 1994), promoting a plant’s ability to survive droughts and to minimise non-stomatal water losses under high temperatures and eVPD (Wahl and Ryser 2000; Terzi et al. 2013; Péli and Nagy-Déri 2018). In this respect, SCP plants represent an ecotype that seems to be better adapted to eVPD (which occasionally might prevail at the plants’ sites during summer months under a sub-Continental climate) than plants originating from AP and SAP (Grant and Hunter 1962; Kuster et al. 2013). On the other hand, SCP plants showed a high chemical trait plasticity in terms of aboveground biomass N contents (and related C:N ratios) in response to eVPD (i.e. increasing C:N rations under eVPD). This finding suggests that plants are capable of increasing the proportion of sclerenchymatic tissue, which in turn would mitigate uncontrolled water losses under eVPD (Wahl and Ryser 2000). Since we did not differentiate between structural and non-structural carbon in our analysis, it is also conceivable that changes in tissue C contents in part might be attributable to the formation of non-structural carbohydrates. However, the bulk of carbon in woody plants is used for sclerenchyma or tissue lignification (Dietze et al. 2013). In addition, the high shoot increment rates found for SCP also may allow for a morphological adjustment to eVPD, since it provides a competitive advantage when recovering from potential aboveground tissue diebacks resulting from drought events or high temperatures (Welch et al. 2006). AP and SAP plants also showed a plastic response of their specific shoot length to eVPD, but specific shoot length values were lowest for SCP (controls). In conclusion, SCP plants were characterised by morphological-chemical traits and (with regard to some traits) a high plasticity, all of which support the plants’ capability to better cope with stressors such as high summer temperatures and a concomitant increase in VPD than plants from AP and SAP.
Although our study revealed a clear response of Calluna plants to eVPD, responses of 1-year old plants to soil drought are much more pronounced (cf. experiments of Meyer-Grünefeldt et al. 2016). This suggests that soil drought proves to be a stronger stressor for young Calluna plants than eVPD. However, as any reduction in summer soil water availability will likely coincide with higher levels of VPD further research on the interaction of these two factors is warranted.
Life-history stage-related susceptibility of Calluna to eVPD
Comparisons of responses of plants from different life-history stages to eVPD confirm our second hypothesis, according to which 1-year old Calluna was expected to be more sensitive to eVPD than 10-year old Calluna. Opposing responses of belowground tissue δ13C signatures of plants from the two life-history stages suggest that 1-year old plants (in tendency) lowered their 13C discrimination, whereas 10-year old plants increased 13C discrimination (likely induced by decreases and increases in stomatal conductance, respectively; Ball and Farquhar 1984).
Comparable to plants originating from different provenances, plants from different life-history stages differed with regard to morphological-chemical traits that co-determine a plant’s sensitivity to atmospheric drought stress (i.e. eVPD). On the one hand, 1-year old plants had a tenfold higher shoot:root ratio than 10-year old plants. Shoot:root ratios are considered a core trait that strongly influences a species’ response to water shortage (Weiner 2004). Relatively high aboveground biomass investments (relative to belowground investments) can foster increasing transpiration rates (Gordon et al. 1999), whereas low belowground biomass allocation (resulting in low root biomass) constrains a plant’s water uptake and thus its water supply (Meyer-Grünefeldt et al. 2016). With progressing life-history stage, Calluna continuously increases its belowground investments, resulting in shoot:root ratios < 0.5 of plants in the mature phase (“apparent plasticity” according to Weiner 2004; also see Gimingham 1972; Wallèn 1987; plantswith a well-established bush-like form). Moreover, older Calluna plants often show a higher rooting depth than young plants, which in turn improves the plants’ access to soil water resources (Meyer-Grünefeldt et al. 2016). In sandy soils (without groundwater influence) the rooting zone of old plants may extend to nearly 50 cm, although often more than 80% of the roots are concentrated in the upper 10 cm of the soil, depending on the thickness of the ecto-organic layer (Gimingham 1960).
The high tissue C:N ratios of 10-year old plants suggest a high proportion of sclerenchymatic tissue accompanied by a high drought tolerance (Van Arendonk and Poorter 1994; Péli and Nagy-Déri 2018). In addition, 10-year old plants exhibited a highly plastic response of this trait to eVPD, indicating that 10-year old plants were able to increase the proportion of aboveground sclerenchymatic tissue in response to eVPD.
In summary, 10-year old plants where characterised by traits indicating a good adaptation to atmospheric (and soil) water stress and thus proved to be less sensitive to eVPD than 1-year old plants. This conclusion is supported by findings of Kongstad et al. (2012) and Meyer-Grünefeldt et al. (2015), according to which mature Calluna plants showed no or little sensitivity to periods of soil drought or high summer temperatures (in terms of aboveground growth depression or tissue δ13C signatures).
Conclusion
In our study, we were able to detect provenance- and life-history stage-specific responses of Calluna plants to eVPD. These findings might have implications for conservation measures in heathland ecosystems under climate change, for example a mitigation of the effects of increasing summer temperatures or decreasing summer precipitation. Although benefits and risks of “assisted migration”-approaches (i.e. the intentional translocation or movement of species outside of their historic ranges to mitigate or counteract climate change-induced losses of species) are controversially debated (Hewitt et al. 2011), it is proven that a high phenotypic plasticity (related to the presence of different ecotypes) will facilitate a population’s resistance or resilience to climate change (Richter et al. 2012). Since Calluna plants originating from SCP revealed several traits that suggest a better adaptation to high summer temperatures or drought events than those from AP or SAP, they principally may constitute an ecotype which is suitable for assisted migration-targets in the context of climate change-mitigation strategies (Rose et al. 2009). However, the intentional translocation of species from donor to new target sites not only needs a justification based on scientific evidence, but also requires a legal framing in the context of an adapted environmental legislation (Pettersson and Keskitalo 2013).
Differences in life-history stage responses to eVPD suggest that the pioneer phase (plant age up to 6 years, before plants have developed a fully bushy habit; Gimingham 1972) constitutes a critical stage within a heathland’s developmental cycle, since 1-year old plants exhibit trait characteristics making them particularly sensitive to high summer temperature or drought events (Meyer-Grünefeldt et al. 2015). In the face of climate change, management strategies such as mowing and prescribed burning may avoid the risk of (extensive) Calluna dieback after drought events. In addition, prescribed burning can facilitate both vegetative and generative regeneration, which in turn may be more favourable for a higher diversity in plant ages per stand.
In contrast, high-intensity measures such as sod-cutting require a generative regeneration (e.g. from the seedbank), with the risk of extensive seedling dieback during drought events. This indicates that management strategies need to be carefully assessed in the context of conceivable trade-offs related to different management targets, for example biodiversity protection, climate change adaptation, compensation of airborne nitrogen inputs, or the protection of landscape multifunctionality.
Availability of data and material
The data and material that support the findings of this study are available from the corresponding author, [KI], upon request.
Change history
19 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11258-021-01195-5
References
Albert KR, Kongstad J, Schmidt IK, Ro-Paulsen H, Mikkelsen TN, Michelsen A, van der Linden L, Beier C (2012) Temperate heath plant responses to dry conditions depends on growth strategy and less on physiology. Acta Oecol 45:79–87. https://doi.org/10.1016/j.actao.2012.09.003
Albert KR, Mikkelsen TN, Michelsen A, Ro-Poulsen H, van der Linden L (2011) Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J Plant Physiol 168:1550–1561. https://doi.org/10.1016/j.jplph.2011.02.011
Ball MC, Farquhar GD (1984) Photosynthetic and stomatal responses of two mangrove species, Aegiceras corniculatum and Avicennia marina, to long term salinity and humidity conditions. Plant Physiol 74:1–6. https://doi.org/10.1104/pp.74.1.1
Collins M, Knutti R, Arblaster J, Dufresne J - L, Fichefet T, Friedlingstein P, Gao X, Gutowski W J, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver A J, Wehner M F, Allen M R, Andrews T, Beyerle U, Bitz C M, Bony S, Booth B B B (2013) Long-term climate change: Projections, commitments and irreversibility. In: Stocker T F, Qin D, Plattner G - K, Tignor M M B, Allen S K, Boschung J, Nauels A, Xia Y, Bex V, Midgley P M (eds) Climate Change 2013—The physical science basis: Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel on Climate Change, Cambridge University Press, New York NY, pp 1029–1136
Cordes H, Kaiser T, von den Lancken H, Lütkepol M, Prüter J (1997) Naturschutzgebiet Lüneburger Heide: Geschichte, Ökologie. Naturschutz, Hausschild, Bremen
Crawley MJ (2007) The R Book. Wiley & Sons, Chichester
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137. https://doi.org/10.1071/PP9820121
Fitter AH (1976) Effects of nutrient supply and competition from other species on root growth of Lolium perenne in soil. Plant Soil 45:177–189. https://doi.org/10.1007/BF00011140
Ford MA, Thorne GN (1974) Effects of atmospheric humidity on plant growth. Ann Bot 38:441–452. https://doi.org/10.1093/oxfordjournals.aob.a084827
Garnier E, Laurent G (1994) Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol 128:725–736. https://doi.org/10.1111/j.1469-8137.1994.tb04036.x
Gimingham CH (1960) Biological flora of the British Isles. Calluna Salisb. A monotypic genus. Calluna vulgaris (L.) Hull. J Ecol 48:455–483. https://doi.org/10.2307/2257528
Gimingham CH (1972) Ecology of heathlands. Chapman & Hall, London
Gobin R, Korboulewsky N, Dumas Y, Balandier P (2015) Transpiration of four common understory plant species according to drought intensity in temperate forests. Ann For Sci 72:1053–1064. https://doi.org/10.1007/s13595-015-0510-9
Gordon C, Woodin SJ, Alexander IJ, Mullins CE (1999) Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytol 142:243–258. https://doi.org/10.1046/j.1469-8137.1999.00399.x
Grant SA, Hunter RF (1962) Ecotypic differentiation of Calluna vulgaris (L.) in relation to altitude. New Phytol 61:44–55. https://doi.org/10.1111/j.1469-8137.1962.tb06272.x
Guerra A, Scremin-Dias E (2018) Leaf traits, sclerophylly and growth habits in plant species of a semiarid environment. Braz J Bot 41:131–144. https://doi.org/10.1007/s40415-017-0416-x
Hewitt N, Klenk N, Smith AL, Bazely DR, Yan N, Wood S, MacLellan JI, Lipsig-Mumme C, Henriques I (2011) Taking stock of the assisted migration debate. Biol Conserv 144:2560–2572. https://doi.org/10.1016/j.biocon.2011.04.031
Hothorn T, Bretz F, Westfall P (2008) Simultaneous interface in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
IPCC (2018) Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. In: Stocker T F, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Cambridge University Press, Cambridge, United Kingdom and New York, NY
Kongstad J, Schmidt IK, Riis-Nielsen T, Arndal MF, Mikkelsen TN, Beier C (2012) High resilience in heathland plants to changes in temperature, drought, and CO2 in combination: results from the CLIMAITE Experiment. Ecosystems 15:269–283. https://doi.org/10.1007/s10021-011-9508-9
Kuster T, Arend M, Günthardt-Georg M, Schulin R (2013) Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 369:61–71. https://doi.org/10.1007/s11104-012-1541-8
Lendzion J, Leuschner C (2008) Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. For Ecol Manag 256:648–655. https://doi.org/10.1016/j.foreco.2008.05.008
Lendzion J, Leuschner C (2009) Temperate forest herbs are adapted to high air humidity—evidence from climate chamber and humidity manipulation experiments in the field. Can J For Res 39:2332–2342. https://doi.org/10.1139/X09-143
Leuschner C (2002) Air humidity as an ecological factor for woodland herbs: leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high vpd levels. Flora 197:262–274. https://doi.org/10.1078/0367-2530-00040
Lihavainen J, Ahonen V, Keski-Saari S, Kontunen-Soppela S, Oksanen E, Keinänen M (2016) Low vapour pressure deficit affects nitrogen nutrition and foliar metabolites of silver birch. J Exp Bot 67:4353–4365. https://doi.org/10.1093/jxb/erw218
Llorens L, Peñuelas J, Beier C, Emmett B, Estiarte M, Tietema A (2004) Effects of an experimental increase of temperature and drought on the photosynthetic performance of two ericaceous shrub species along a north-south European gradient. Ecosystems 7:613–624. https://doi.org/10.1007/s10021-004-0180-1
Loidi J, Biurrun I, Campos JA, García-Mijangos I, Herrera M (2010) A biogeological analysis of the European Atlantic lowland heathlands. J Veg Sci 21:832–842. https://doi.org/10.1111/j.1654-1103.2010.01204.x
May W, Ganske A, Leckebusch GC, Rockel B, Tinz B, Ulbrich U (2016) Projected change—atmosphere. In: Quante M, Colijn F (eds) North Sea Region Climate Change Assessment. Regional Climate Studies. Springer, Cham, pp 149–173
Meyer-Grünefeldt M, Belz K, Calvo L, Marcos E, von Oheimb G, Härdtle W (2016) Marginal Calluna populations are more resistant to climate change, but not under high-nitrogen loads. Plant Ecol 217:111–122. https://doi.org/10.1007/s11258-015-0563-8
Meyer-Grünefeldt M, Calvo L, Marcos E, von Oheimb G, Härdtle W (2015) Impacts of drought and nitrogen addition on Calluna heathlands differ with plant life-history stage. J Ecol 103:1141–1152. https://doi.org/10.1111/1365-2745.12446
Monteith JL, Unsworth MH (2008) Principles of environmental physics: plants, animals, and the atmosphere. Academic Press, Burlington
Ocheltree TW, Nippert JB, Prasad PVV (2014) Stomatal responses to changes in vapour pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant Cell Environ 37:132–139. https://doi.org/10.1111/pce.12137
O’Gorman PA, Muller CJ (2010) How closely do changes in surface and column water vapor follow Clausius-Clapeyron scaling in climate change simulations? Environ Res Lett 5:025207. https://doi.org/10.1088/1748-9326/5/2/025207
Péli ER, Nagy-Déri H (2018) Different morpho-anatomical strategies against desiccation in five species of Xerophyta genus in relation to their ecophysiological aspects. S Afr J Bot 118:232–240. https://doi.org/10.1016/j.sajb.2018.07.030
Peñuelas J, Gordon C, Llorens L, Nielsen T, Tietema A, Beier C, Bruna P, Emmett B, Estiarte M, Gorissen A (2004) Nonintrusive field experiments show different plant responses to warming and drought among sites, seasons, and species in a North-South European gradient. Ecosystems 7:598–612. https://doi.org/10.1007/s10021-004-0179-7
Pettersson M, Keskitalo ECH (2013) Adaptive capacity of legal and policy frameworks for biodiversity protection considering climate change. Land Use Policy 34:213–222. https://doi.org/10.1016/j.landusepol.2013.03.007
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2010) nlme: linear and nonlinear mixed effects models. R package version 3.1 – 143. https://CRAN.R-project.org/package=nlme
R Core Team (2019) R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. https://www.R-project.org/
Richter S, Kipfer T, Wohlgemuth T, Calderón Guerrero C, Ghazoul J, Moser B (2012) Phenotypic plasticity facilitates resistance to climate change in a highly variable environment. Oecologia 169:269–279. https://doi.org/10.1007/s00442-011-2191-x
Rose L, Leuschner C, Köckemann B, Buschmann H (2009) Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur J For Res 128:335–343. https://doi.org/10.1007/s10342-009-0268-4
Sadok W, Sinclair T (2010) Genetic variability of transpiration response of soybean [Glycine max (L.) Merr.] shoots to leaf hydraulic conductance inhibitor AgNO3. Crop Sci 50:1423–1430. https://doi.org/10.2135/cropsci2009.10.0575
Terzi R, Saruhan Güler N, Kutlu Çalişkan N, Kadioğlu A (2013) Lignification response of rolled leaves of Ctenanthe setosa under long-term drought stress. Turk J Biol 37:614–619. https://doi.org/10.3906/biy-1210-27
Thomas TH, Davies I (2002) Responses of dormant heather (Calluna vulgaris) seeds to light, temperature, chemical and advanced treatments. Plant Growth Regul 37:23–29. https://doi.org/10.1023/A:1020396112716
Tibbitts TW (1979) Humidity and plants. Bioscience 29:358–363. https://doi.org/10.2307/1307692
Van Arendonk JJCM, Poorter H (1994) The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant Cell Environ 17:963–970. https://doi.org/10.1111/j.1365-3040.1994.tb00325.x
von Oheimb G, Power SA, Falk K, Friedrich U, Mohamed A, Krug A, Boschatzke N, Härdtle W (2010) N: P ratio and the nature of nutrient limitation in Calluna-dominated heathlands. Ecosystems 13:317–327. https://doi.org/10.1007/s10021-010-9320-y
Wagner S, Berg P, Schädler G, Kunstmann H (2013) High resolution regional climate model simulations for Germany: Part II-projected climate changes. Clim Dyn 40:415–427. https://doi.org/10.1007/s00382-012-1510-1
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148:459–471. https://doi.org/10.1046/j.1469-8137.2000.00775.x
Wallèn B (1987) Growth pattern and distribution of biomass of Calluna vulgaris on an ombrotrophic peat bog. Ecography 10:73–79. https://doi.org/10.1111/j.1600-0587.1987.tb00741.x
Watt AS (1947) Pattern and processes in the plant community. J Ecol 35:1–22. https://doi.org/10.2307/2256497
Watt AS (1955) Bracken versus heather, a study in plant sociology. J Ecol 43:490–506. https://doi.org/10.2307/2257009
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol 6:207–215. https://doi.org/10.1078/1433-8319-00083
Welch D, Scott D, Mitchell R, Elston DA (2006) Slow recovery of heather (Calluna vulgaris L. (Hull)) in Scottish moorland after easing of heavy grazing pressure from red deer (Cervus elaphus L.). Bot J Scotl 58:1–17. https://doi.org/10.1080/03746600608685103
Wilson PJ, Thompson K, Hodgson JG (1999) Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol 143:155–162. https://doi.org/10.1046/j.1469-8137.1999.00427.x
WMO (2008) Guide to meteorological instruments and methods of observation, No. 8, World Meteorological Organization, Geneva
Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282:242–246. https://doi.org/10.1038/282424a0
Zhang C, Yang H, Wu W, Li W (2017) Effect of drought stress on physiological changes in leaf surface morphology in the blackberry. Braz J Bot 40:625–634. https://doi.org/10.1007/s40415-017-0377-0
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We are grateful to the Untere Naturschutzbehörde Bad Fallingbostel for collaboration and the provision of heathland sites for the set-up of our experiment. We also thank Markus Hilscher for providing the infrastructure needed for our experiment. We are grateful to Prof. Dr. Vigdis Vandvik from the University of Bergen for the organisation of the seed collection at Lygra Heath in Norway and to the DBU Naturerbe GmbH for the permission to collect seeds in the Oranienbaum Heath. The first author was funded by a doctoral scholarship from the German Federal Environmental Foundation (DBU; AZ 20015/392).
Funding
Open Access funding enabled and organized by Projekt DEAL. KI was funded by a doctoral scholarship from the German Federal Environmental Foundation (DBU; AZ 20015/392).
Author information
Authors and Affiliations
Contributions
CL, WH and HC contributed to the study conception and design. Material preparation and data collection were performed by HC and KI. Data analysis was performed by AF and KI. The first draft of the manuscript was written by KI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Marjan Jongen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibe, K., Walmsley, D., Fichtner, A. et al. Provenance- and life-history stage-specific responses of the dwarf shrub Calluna vulgaris to elevated vapour pressure deficit. Plant Ecol 221, 1219–1232 (2020). https://doi.org/10.1007/s11258-020-01076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01076-3