Abstract
Summary
We investigated the effect of 12 months of functional electrical stimulation–assisted rowing with and without zoledronic acid (ZA) on computationally estimated bone strength and stiffness in individuals with spinal cord injury. We found that rowing with ZA, but not rowing alone, improved stiffness at the distal femur, but not the proximal tibia.
Introduction
People with spinal cord injury (SCI) have high fracture risk at the knee after the injury. Therapies that prevent bone loss or stimulate an anabolic response in bone have been proposed to reduce fractures. Zoledronic acid (ZA) is a potent bisphosphonate that inhibits osteoclastic resorption. Functional electrical stimulation (FES)–assisted rowing is a potentially osteogenic exercise involving mechanical stimulation to the lower extremities. Here, we investigated the effect of FES-assisted rowing with and without ZA on bone strength and stiffness in individuals with SCI.
Methods
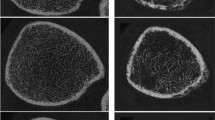
Twenty individuals from a cohort of adults with SCI who participated in a clinical trial were included in the study. CT scans of their knees before and after the intervention were converted to finite element models. Bone failure strength (Tult) and stiffness were calculated at the proximal tibia and distal femur.
Results
Tult at the distal femur increased 4.6% among people who received rowing + ZA and decreased 13.9% among those with rowing only (p < 0.05 for group). Torsional and compressive stiffness at the femur metaphysis increased in people with rowing + ZA (+ 3 to +4%) and decreased in people with rowing only (− 7 to −8%; p < 0.05). Tult in the proximal tibia decreased in everyone, but the loss was attenuated in the rowing + ZA group. People with initially stronger bone tended to lose more strength.
Conclusion
Overall, we observed increases in bone strength at the distal femur but not the proximal tibia, with FES–assisted rowing combined with ZA treatment. Rowing alone did not significantly prevent bone loss at either site, which might be attributed to insufficient mechanical loading.


Similar content being viewed by others
Data availability
Public clinical trial registration: http://clinicaltrials.gov/show/NCT01426555. FES-Rowing Versus Zoledronic Acid to Improve Bone Health in Spinal Cord Injury (SCI).
References
Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, Kraenzlin M, Zach G, Lippuner K (2004) Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 15:180–189
Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E (2009) Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int 20:385–392
Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA (2017) Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int 28:747–765
Rubin C, Xu G, Judex S (2001) The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 15:2225–2229
Edwards WB, Simonian N, Troy KL, Schnitzer TJ (2015) Reduction in torsional stiffness and strength at the proximal tibia as a function of time since spinal cord injury. Journal of bone and mineral research
Troy KL, Morse LR (2015) Measurement of bone: diagnosis of SCI-induced osteoporosis and fracture risk prediction. Top Spinal Cord Inj Rehabil 21:267–274
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM (2015) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab 33:410–421
Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, Caminis J (2007) Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int 80:316–322
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW (2011) Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 22:271–279
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D (2016) Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo-controlled pilot trial. PM & R : the journal of injury, function, and rehabilitation 8:833–843
Pearson EG, Nance PW, Leslie WD, Ludwig S (1997) Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil 78:269–272
Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R, Kraenzlin M, Zach GA, Lippuner K (2004) Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 19:1067–1074
Fuchs RK, Shea M, Durski SL, Winters-Stone KM, Widrick J, Snow CM (2007) Individual and combined effects of exercise and alendronate on bone mass and strength in ovariectomized rats. Bone 41:290–296
Bloomfield SA, Mysiw WJ, Jackson RD (1996) Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 19:61–68
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson Nde N, Eser P (2008) High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 43:169–176
Lambach RL, Stafford NE, Kolesar JA, Kiratli BJ, Creasey GH, Gibbons RS, Andrews BJ, Beaupre GS (2018) Bone changes in the lower limbs from participation in an FES rowing exercise program implemented within two years after traumatic spinal cord injury. J spinal cord med 1-9
Morse LR, Troy KL, Fang Y, Nguyen N, Battaglino R, Goldstein RF, Gupta R, Taylor JA (2019) Combination therapy with zoledronic acid and FES-row training mitigates bone loss in paralyzed legs: results of a randomized comparative clinical trial. JBMR Plus 3:e10167
Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML (2011) Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res 26:974–983
Borggrefe J, Graeff C, Nickelsen TN, Marin F, Gluer CC (2010) Quantitative computed tomographic assessment of the effects of 24 months of teriparatide treatment on 3D femoral neck bone distribution, geometry, and bone strength: results from the EUROFORS study. J Bone Miner Res 25:472–481
Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM (2008) Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res 23:1974–1982
Edwards WB, Schnitzer TJ, Troy KL (2013) Torsional stiffness and strength of the proximal tibia are better predicted by finite element models than DXA or QCT. J Biomech 46:1655–1662
Edwards WB, Schnitzer TJ, Troy KL (2013) Bone mineral loss at the proximal femur in acute spinal cord injury. Osteoporos Int 24:2461–2469
Edwards WB, Schnitzer TJ, Troy KL (2014) Reduction in proximal femoral strength in patients with acute spinal cord injury. J Bone Miner Res 29:2074–2079
Edwards WB, Schnitzer TJ, Troy KL (2014) Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos Int 25:1005–1015
Edwards WB, Schnitzer TJ, Troy KL (2014) The mechanical consequence of actual bone loss and simulated bone recovery in acute spinal cord injury. Bone 60:141–147
Rho JY (1996) An ultrasonic method for measuring the elastic properties of human tibial cortical and cancellous bone. Ultrasonics 34:777–783
Rho JY, Hobatho MC, Ashman RB (1995) Relations of mechanical properties to density and CT numbers in human bone. Med Eng Phys 17:347–355
Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM (2004) Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech 37:27–35
Gupta A, Bayraktar HH, Fox JC, Keaveny TM, Papadopoulos P (2007) Constitutive modeling and algorithmic implementation of a plasticity-like model for trabecular bone structures. Comput Mech 40:61–72
Hill R (1948) A theory of the yielding and plastic flow of anisotropic metals. Proceedings of the Royal Society of London Series A, Mathematical and Physical Sciences 193:281–297
Rincon-Kohli L, Zysset PK (2009) Multi-axial mechanical properties of human trabecular bone. Biomech Model Mechanobiol 8:195–208
Gray HA, Taddei F, Zavatsky AB, Cristofolini L, Gill HS (2008) Experimental validation of a finite element model of a human cadaveric tibia. J Biomech Eng 130:031016
Anastasilakis AD, Polyzos SA, Efstathiadou ZA, Savvidis M, Sakellariou GT, Papatheodorou A, Kokkoris P, Makras P (2015) Denosumab in treatment-naive and pre-treated with zoledronic acid postmenopausal women with low bone mass: effect on bone mineral density and bone turnover markers. Metab Clin Exp 64:1291–1297
McPherson JG, Edwards WB, Prasad A, Troy KL, Griffith JW, Schnitzer TJ (2014) Dual energy X-ray absorptiometry of the knee in spinal cord injury: methodology and correlation with quantitative computed tomography. Spinal Cord 52:821–825
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RLM, Tan M, Ruzycky ME, Su G, Eastell R (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT). J Bone Miner Res 27:243–254
Varghese SM, Chandy BR, Thomas R, Tharion G (2016) Effect of zoledronic acid on osteoporosis after chronic spinal cord injury: a randomized controlled trial. 28:85–93
Dudley-Javoroski S, Shields RK (2013) Active-resisted stance modulates regional bone mineral density in humans with spinal cord injury. The journal of spinal cord medicine 36:191–199
Groah SL, Lichy AM, Libin AV, Ljungberg I (2010) Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PM & R : the journal of injury, function, and rehabilitation 2:1080–1087
Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Leiby BE, Sendecki J, Singh H, Modlesky CM (2016) Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: a pilot study. Arch Phys Med Rehabil 97:1413–1422
Gibbs JC, Craven BC, Moore C, Thabane L, Adachi JD, Giangregorio LM (2015) Muscle density and bone quality of the distal lower extremity among individuals with chronic spinal cord injury. Top Spinal Cord Inj Rehabil 21:282–293
Fang Y (2018) Investigation of bone loading in FES-assisted rowing and its effect on preventing bone loss in people with spinal cord injury. Biomedical engineering. Worcester Polytechnic Institute, Worcester, MA, p 138
Draghici AE, Picard G, Taylor JA, Shefelbine SJ (2017) Assessing kinematics and kinetics of functional electrical stimulation rowing. J Biomech 53:120–126
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34:869–880
Draghici AE, Taylor JA, Bouxsein ML, Shefelbine SJ (2019) Effects of FES-rowing exercise on the time-dependent changes in bone microarchitecture after spinal cord injury: a cross-sectional investigation. JBMR Plus 3:e10200
Hunter JG, Garcia GL, Shim JK, Miller RH (2019) Fast running does not contribute more to cumulative load than slow running. Med Sci Sports Exerc 51:1178–1185
Troy KL, Mancuso ME, Johnson JE, Wu Z, Schnitzer TJ, Butler TA (2020) Bone adaptation in adult women is related to loading dose: a 12-month randomized controlled trial. J Bone Miner Res 35:1300–1312
Hernandez CJ, Beaupré GS, Marcus R, Carter DR (2001) A theoretical analysis of the contributions of remodeling space, mineralization, and bone balance to changes in bone mineral density during alendronate treatment. Bone 29:511–516
Rodan GA, Fleisch HA (1996) Bisphosphonates: mechanisms of action. J Clin Investig 97:2692–2696
Brouwers JE, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R (2008) Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcif Tissue Int 82:202–211
Lespessailles E, Jaffre C, Beaupied H, Nanyan P, Dolleans E, Benhamou CL, Courteix D (2009) Does exercise modify the effects of zoledronic acid on bone mass, microarchitecture, biomechanics, and turnover in ovariectomized rats? Calcif Tissue Int 85:146–157
Dragomir-Daescu D, Salas C, Uthamaraj S, Rossman T (2015) Quantitative computed tomography-based finite element analysis predictions of femoral strength and stiffness depend on computed tomography settings. J Biomech 48:153–161
Taylor JA, Picard G, Widrick JJ (2011) Aerobic capacity with hybrid FES rowing in spinal cord injury: comparison with arms-only exercise and preliminary findings with regular training. PM & R : the journal of injury, function, and rehabilitation 3:817–824
Funding
This study received support from the Department of Defense (W81XWH-10-1-1043 to LRM).
Author information
Authors and Affiliations
Contributions
Parent study conceived by LRM and RAB. Analysis conceived by KLT and LRM. Analysis performed by YF, with contributions from NN and RFG. Data interpretation by LRM, RAB, and KLT. Manuscript drafted by YF and KLT with input from all authors.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Consent to participate
The present study was a secondary analysis that utilized deidentified data only. It did not require institutional approval. The parent study was institutionally approved, and all participants gave written informed consent prior to participation.
Code availability
Available upon reasonable request.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Fang, Y., Morse, L., Nguyen, N. et al. Functional electrical stimulation (FES)–assisted rowing combined with zoledronic acid, but not alone, preserves distal femur strength and stiffness in people with chronic spinal cord injury. Osteoporos Int 32, 549–558 (2021). https://doi.org/10.1007/s00198-020-05610-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05610-x