Article contents
Thermodynamic properties of rare-earth alloys by electrochemical emf measurements
Published online by Cambridge University Press: 03 September 2020
Abstract
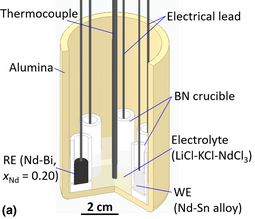
Thermodynamic properties of Nd–Bi and Nd–Sn alloys were determined via electromotive force (emf) measurements at 725–1075 K. The emf measurements of an Nd–Bi alloy at mole fraction xNd = 0.20 were conducted using a solid CaF2–NdF3 electrolyte relative to pure Nd(s). The emf values from the CaF2–NdF3 electrolyte were verified in separate experiments in molten LiCl–KCl–NdCl3 where pure Nd(s) was electrodeposited. The Nd–Bi (xNd = 0.20) exhibited two-phase behavior with a peritectic reaction (L + NdBi = NdBi2) at 926 K from differential scanning calorimetry. The two-phase Nd–Bi (xNd = 0.20) was employed as a stable reference electrode in molten LiCl–KCl–NdCl3 for emf measurements of Nd–Bi (xNd = 0.15–0.40) and Nd–Sn (xNd = 0.10) alloys. The emf measurements of these alloys were reproducible during thermal cycles over 50 h and were used to calculate thermodynamic properties, including the partial molar Gibbs energy, entropy, and enthalpy.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 9
- Cited by





