Abstract
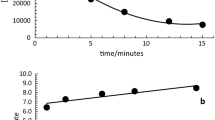
The sorption and desorption of a mixture of phenolic acids by the kaolinite modified with aluminum hydroxide was studied in the presence of acetate buffer. The experiments were performed in a 1-mL flow-through microcolumn in 5 and 50 mM sodium acetate buffer (pH 4.5) at a flow rate of 0.5 mL/min. The total concentration of phenolic acids was 0.01 mg/mL (0.06 mM); the concentration of each acid was 0.01 mM. Sodium acetate buffer (5 and 50 mM, pH 4.5) and 50 mM sodium acetate buffer supplemented with 0.1 mM oxalic acid were used for desorption. The concentration of phenolic acids was determined by reverse phase high-pressure liquid chromatography. The following order of sorption was observed: gallic > protocatechuic \( \gg \)p-hydroxybenzoic ~ vanillic ~ ferulic ~ syringic acid. The sorption of phenolic acids in 50 mM buffer comprised 18–35% of their sorption in 5 mM buffer, suggesting the competition of acetate ions and phenolic acids for binding sites on the mineral. The order of desorption of phenolic acids was opposite to the order of sorption and generally correlated with the stability constants of the phenolic acid complexes with aluminum hydroxide. All acids, except for gallic and protocatechuic acids, were weakly bound to the mineral and were almost completely (88–98%) desorbed with 5 mM acetate buffer. The total desorption of gallic and protocatechuic acids by all eluents comprised 25 and 45% of their sorbed amount, respectively. Desorption was significant only in 50 mM acetate buffer (12 and 23%) and in the same buffer with 0.1 mM oxalic acid (10 and 15%). Thus, it has been shown that the distribution of phenolic acids between the solid phase and solution is largely determined by the presence of competing aliphatic compounds. Based on desorption experiments, we propose possible types of complexes of phenolic acids and aluminum hydroxide on the surface of the mineral.




Similar content being viewed by others
REFERENCES
M. S. Ermolin, N. N. Fedyunina, V. K. Karandashev, and P. S. Fedotov, “Study of the mobility of cerium oxide nanoparticles in soil using dynamic extraction in a microcolumn and a rotating coiled column,” J. Anal. Chem. 74, 825–833 (2019). https://doi.org/10.1134/S1061934819080070
A. G. Zavarzina, M. S. Ermolin, V. V. Demin, and P. S. Fedotov, “Interaction of the mixture of phenolic acids with modified kaolinite under batch and dynamic conditions,” Eurasian Soil Sci. 51, 938-946 (2018). https://doi.org/10.1134/S1061934819080070
E. I. Karavanova, “Dissolved organic matter: fractional composition and sorbability by the soil solid phase (review of literature),” Eurasian Soil Sci. 46, 833-844 (2013). https://doi.org/10.1134/S1064229313080048
M. S. Malinina and S. V. Ivanilova, “Phenol compounds in solutions of soils of different types in the central forest state biosphere reserve,” Eurasian Soil Sci. 41, 377–385 (2008). https://doi.org/10.1134/S1064229308040030
E. V. Shamrikova, V. V. Punegov, I. V. Gruzdev, E. V. Vanchikova, and A. A. Vetoshkina, “Individual organic compounds in water extracts from podzolic soils of the Komi Republic,” Eurasian Soil Sci. 45, 939–947 (2012). https://doi.org/10.1134/S1064229312100080
E. V. Shamrikova, O. S. Kubik, S. V. Deneva, and V. V. Punegov, “Composition of the water-soluble soil fraction on the Barents Sea coast: organic carbon and nitrogen, low-molecular weight components,” Eurasian Soil Sci. 52, 1347–1362 (2019). https://doi.org/10.1134/S1064229319110103
M. L. Adams, B. O’Sullivan, A. J. Downard, and K. J. Powell, “Stability constants for aluminum(III) complexes with the 1,2-dihydroxyaryl ligands caffeic acid, chlorogenic acid, DHB, and DASA in aqueous solution,” J. Chem. Eng. Data 47, 289–296 (2002).
R. Adeleke, C. Nwangburuka, and B. Oboiriend, “Origins, roles and fate of organic acids in soils: a review,” South Afr. J. Bot., (2016). https://doi.org/10.1016/j.sajb.2016.09.002
V. J. Alstadt, J. D. Kubicki, and M. A. Freedman, “Competitive adsorption of acetic acid and water on kaolinite,” J. Phys. Chem. A 120 (42), 8339–8346 (2016). https://doi.org/10.1021/acs.jpca.6b06968
A. Beneduci, E. Furia, N. Russo, and T. Marino, “Complexation behaviour of caffeic, ferulic and p-coumaric acids towards aluminium cations: a combined experimental and theoretical approach,” New J. Chem. 41, 5182 (2017).
A. M. Cecchi, W. C. Koskinen, H. H. Cheng, and K. Haider, “Sorption-desorption of phenolic acids as affected by soil properties,” Biol. Fertil. Soils 39, 235–242 (2004).
R. Croteau, T. M. Kutchan, and N. G. Lewis, “Natural products (secondary metabolites),” in Biochemistry and Molecular Biology of Plants, Ed. by B. Buchanan, (American Society of Plant Physiologists, Rockville, 2000), pp. 1250–1318.
C. Evanko and D. Dzombak, “Influence of structural features on sorption of NOM-analogue organic acids to goethite,” Environ. Sci. Technol. 32, 2846–2855 (1998).
J. Gao, B. Jansen, R. Cerli, A. Helmus, R. Mikutta, S. Dultz, G. Guggenberger, and K. Kalbitz, “Competition and surface conditioning alter the adsorption of phenolic and amino acids on soil minerals,” Eur. J. Soil Sci. 68 (5), 667–677 (2017). https://doi.org/10.1111/ejss.12459
J. Gao, B. Jansen, R. Cerli, A. Helmus, R. Mikutta, S. Dultz, G. Guggenberger, C. Vogel, and K. Kalbitz, “Organic matter coatings of soil minerals affect adsorptive interactions with phenolic and amino acids,” Eur. J. Soil Sci. 69 (4), 613–624 (2018). https://doi.org/10.1111/ejss.12562
S. Goldberg, J. A. Davis, and J. D. Hem, “The surface chemistry of aluminum oxides and hydroxides,” in The Environmental Chemistry of Aluminum, Ed. by G. Sposito (CRC Press, Boca Raton, FL, 1996), pp. 271–332.
B. Gu, T. L. Mehlhorn, L. Liang, and J. F. McCarthy, “Competitive adsorption, displacement, and transport of organic matter on iron oxide: II. Displacement and transport,” Geochim. Cosmochim. Acta 60 (16), 2977–2992 (1996).
X. H. Guan, C. Shang, and G.-H. Chen, “ATR-FTIR investigation of the role of phenolic groups in the interaction of some NOM model compounds with aluminum hydroxide,” Chemosphere 65, 2074–2081 (2006).
K. Kaiser and G. Guggenberger, “The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils,” Org. Geochem. 31, 711–725 (2000).
K. Kalbitz, D. Schwesig, J. Rethemeyer, and E. Matzner, “Stabilization of dissolved organic matter by sorption to the mineral soil,” Soil Biol. Biochem. 37, 1319–1331 (2005).
M. Kleber, K. Eusterhues, M. Keiluweitk, C. Mikutta, R. Mikutta, and P. S. Nico, “Mineral-organic associations: formation, properties, and relevance in soil environments,” in Advances in Agronomy (Elsevier, Amsterdam, 2015), Vol. 130, pp. 1–140.
I. Kögel-Knabner, G. Guggenberger, M. Kleber, E. Kandeler, K. Kalbitz, S. Scheu, et al., “Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry,” J. Plant Nutr. Soil Sci. 171, 61–82 (2008).
J. Lewis and J. Sjöstrom, “Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments,” J. Contam. Hydrol. 115, 1 (2010).
M. A. Olofsson, S. H. Norström, and D. Bylund, “Evaluation of sampling and sample preparation procedures for the determination of aromatic acids and their distribution in a podzol soil using liquid chromatography-tandem mass spectrometry,” Geoderma 23, 373–380 (2014).
T. Polubesova, S. Eldad, and B. Chefetz, “Adsorption and oxidative transformation of phenolic acids by Fe(III)-montmorillonite,” Environ. Sci. Technol. 44, 4203–4209 (2010).
M. W. I. Schmidt, M. S. Torn, S. Abiven, T. Dittmar, G. Guggenberger, I. A. Janssens, et al., “Persistence of soil organic matter as an ecosystem property,” Nature 478, 49–56 (2011).
The Environmental Chemistry of Aluminum, Ed. by G. Sposito (CRC Press, Boca Raton, FL, 1996).
G. Sposito, The Chemistry of Soil (Oxford University Press, Oxford, 2008).
W. Stepniewski, R. Dudzinska, and L. Pawlowski, “Aluminium transport in soil with particular emphasis on the role of organic matter,” J. Ecol. Chem. 3 (3), 195–232 (1994).
N. Tharayil, P. C. Bhowmik, and B. Xing, “Preferential sorption of phenolic phytotoxins to soil: implications for altering the availability of allelochemicals,” J. Agric. Food Chem. 54, 3033–3040 (2006).
Funding
The work was supported by the Russian Science Foundation (grant no. 17-14-01207).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by G. Chirikova
Rights and permissions
About this article
Cite this article
Zavarzina, A.G., Ermolin, M.S., Demin, V.V. et al. The Effect of Acetic Acid and Acetate Ions on Sorption–Desorption of a Mixture of Phenolic Acids by Modified Kaolinite. Eurasian Soil Sc. 53, 1046–1055 (2020). https://doi.org/10.1134/S1064229320080177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229320080177