A Dual Swine Challenge With Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae Used to Compare a Combination of Mixable Monovalent PCV2 and Monovalent M. hyopneumoniae Vaccines With a Ready-to Use PCV2 and M. hyopneumoniae Bivalent Vaccine
- Department of Veterinary Pathology, College of Veterinary Medicine, Gwanak-ro 1, Gwanak-gu, Seoul National University, Seoul, South Korea
The present study evaluated the efficacy of swine vacciation using a combination of mixable monovalents for porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae against a ready-to-use bivalent vaccine under experimental conditions. Pigs at 21 days of age were administered either a combination of two mixable monovalent vaccines or a bivalent vaccine containing PCV2 and M. hyopneumoniae. Vaccination was followed with an M. hyopneumoniae challenge at 42 days of age (−14 days post challenge, dpc) and a PCV2d challenge at 56 days of age (0 dpc). Each vaccinated and challenged group was compared with the unvaccinated and challenged group for clinical, microbiological, immunologic, and pathologic differences. Clinically, two vaccinated and challenged groups showed minimal respiratory diseases that was characterized by occasionally coughing and sneezing. A significant difference was not calculated in the average daily weight gain, nasal shedding of M. hyopneumoniae, and pathological lesions between two vaccinated and challenged groups. A combination of two monovalent vaccines mixed into a combo prior to vaccination followed by challenge resulted in increased numbers of PCV2d-specific interferon-γ secreting cells at 21 dpc and a significant reduction in PCV2d viremia at 14 dpc when compared with the ready-to-use bivalent-vaccinated and challenged groups. These results offer supporting evidence that vaccination during the weaning to finishing period against M. hyopneumoniae and PCV2 is efficacious for controlling diseases caused by these two pathogens.
Introduction
Porcine respiratory disease complex (PRDC) is one of the most important swine health concerns to producers today. PRDC is most prevalent in large swine farms that implement a continuous production system, often containing around 6–20 weeks-old pigs (1, 2). Common clinical symptoms of PRDC include growth retardation, decreased feed efficiency, anorexia, lethargy, fever, cough, and dyspnea.
Porcine circovirus type 2 (PCV2) is the smallest non-enveloped, single-stranded, circular DNA virus within the genus Circovirus and the family Circoviridae (3). Currently, PCV2 is classified with six different genotypes, a-f (4–7) with PCV2d prevailing as the most frequent genotype currently circulating within the US and Korea (8, 9). PCV2 is the causative agent of PCV2-systemic disease as well as various other circovirus-related syndromes (10–12).
Mycoplasma hyopneumoniae bacterium is one of the most important pathogens within the PRDC, and causes chronic respiratory conditions in pigs as the etiologic agent of enzootic pneumonia. Dry, non-productive coughing, and growth retardation are the most obvious clinical signs, although the majority of M. hyopneumoniae infections are subclinical (13).
PCV2 and M. hyopneumoniae are both known to affect swine herds worldwide. PRDC development is often caused by a co-infection of these two pathogens (14). This in turn has resulted in an increase in animal treatment costs as well as in economic losses. Vaccination within the Asian pork industry remains the most common and effective tool in controlling PCV2 and M. hyopneumoniae infection. The recommended times of vaccination against PCV2 and M. hyopneumoniae are similar so the use of either a combination of two monovalent vaccines mixed into a combo prior to vaccination, or a ready-to-use combination vaccine containing PCV2 and M. hyopneumoniae are growing in popularity. The objective of this study was to compare the efficacy of a combination of two monovalent vaccines mixed into a combo prior to vaccination with a ready-to-use bivalent vaccine containing PCV2 and M. hyopneumoniae. The vaccines were evaluated with a dual M. hyopneumoniae and PCV2d challenge in pigs and conclusions were based on clinical, microbiological, immunological, and pathological outcomes.
Materials and Methods
Ethical Statement
This study was approved by the Seoul National University Institutional Animal Care and Use Committee (SNU-190712-5).
Animals
Piglets were selected from commercial farms which tested free of porcine reproductive and respiratory syndrome virus (PRRSV) and M. hyopneumoniae based on breeding herd serology, and long term clinical and slaughter history. A total of 60 colostrum-fed, cross-bred, conventional piglets were purchased at 7 days old from the selected facilities. Upon arrival, piglets were tested for PCV2 and PRRSV viremia as well as for nasal shedding of M. hyopneumoniae by real-time polymerase chain reaction (PCR). Piglets were also tested and confirmed seronegative for PCV2 (SERELISA PCV2 Ab Mono Blocking, Synbiotics, Lyon, France), PRRSV (HerdCheck PRRS X3 Ab test, IDEXX Laboratories Inc., Westbrook, ME, USA), and M. hyopneumoniae (M. hyo. Ab test, IDEXX Laboratories Inc.), according to routine serological testing.
Experimental Design
The random number generator function (Excel, Microsoft Corporation, Redmond, Washington, USA) was used to randomly divide 60 pigs into 4 groups, each containing 15 pigs (Table 1). The minimum sample size per each group was calculated as suggested by Cohen (15) using pwr package in R v.3.5.1 (R Core Team: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). The minimum number of piglets needed per group was calculated using a 0.05 significance level, 0.4 effect size, and 70% power. Under these parameters, the minimum number of pigs per group equated to 14.69, therefore, at least 15 piglets were designated per group. Three groups of pigs, identified as VacA/Ch, VacB/Ch, and UnVac/Ch, were randomly assigned into 3 rooms. Pigs in an additional UnVac/UnCh group were randomly assigned into 1 room. Each room uniformly constained 2 pens (7 or 8 pigs per pen) and each pig was assigned a pen randomly using the random number generator function (Excel, Microsoft Corporation). Rooms and pens were consistent in design and were equipped with equipped with free-access to water and feed throughout the duration of the study.
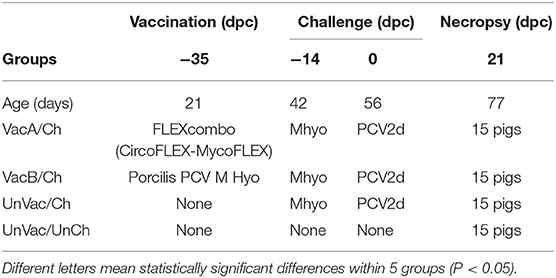
Table 1. Experimental design with vaccination and challenge strategies for Mycoplasma hyopneumoniae (Mhyo) and porcine circovirus type 2d (PCV2d) at different days post-challenge (dpc).
Pigs in the VacA/Ch group were vaccinated intramuscularly with FLEXcombo (Boehringer Ingelheim Vetmedica Inc., St. Joseph, Missouri, USA) at 21 days of age in accordance with the manufacturer's directions. FLEXcombo vaccine was prepared by mixing equal volumes of Ingelvac CiroFLEX (Serial No. 3091282A) and Ingelvac MycoFLEX (Serial No. 273052A). Each pigs in VacA/Ch group received a 2 mL/dose of freshly mixed FLEXcombo vaccine. Pigs in VacB/Ch group were intramuscularly vaccinated with a 2.0 mL dose of the ready-to-use combination vaccine Porcilis PCV M Hyo (Lot No. C530B01, MSD Animal Health, Boxmeer, Netherlands), also at 21 days of age. UnVac/Ch and UnVac/UnCh pig groups were administered a 2.0 mL dose of phosphate buffered saline (PBS, 0.01M, pH 7.4) at 21 days old.
Pigs in the VacA/Ch, VacB/Ch, and UnVac/Ch groups were anesthetized at −14 days post challenge (dpc, 42 days old), with a mixture of 2.2 mg/kg xylazine hydrochloride (Rumpon, Bayer Korea, Seoul, Korea), 2.2 mg/kg tiletamine hydrochloride, and 2.2 mg/kg zolazepam hydrochloride (Zoletil 50, Virbac Korea, Seoul, Korea) by intramuscular injection. Post-anesthetization, they were immediately inoculated with an intratracheal challenge containing 7 mL of M. hyopneumoniae culture medium with 107 color changing units (CCU)/mL as described previously (16, 17). VacA/Ch, VacB/Ch, and UnVac/Ch pig groups were inoculated intranasally with 3 mL of tissue culture supernatant containing 1.2 × 105 TCID50/mL of PCV2d (SNUVR140004, GenBank no. KJ437506) at 0 dpc (56 days old) (12).
Blood and nasal swabs were collected at the following time points: −49 (7 days old), −35 (21 days old), −7 (42 days old), 0 (56 days old), 7 (63 days old), 14 (70 days old), and 21 (77 days old) dpc. An intravenous injection of sodium pentobarbital was used to sedate all pigs prior to euthanization by electrocution at 21 dpc as previously described (18).
Clinical Observations
Pigs were monitored daily for clinical signs and scored weekly using a score ranking system which ranged from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (19). All observers involved in these processes were blinded to vaccination and type of vaccine status. At the end of the study, mortality rate was calculated for each group. Mortality rate was defined as the number of pigs that died divided by the number of pigs initially assigned to that group within the batch. All pigs that died or were culled throughout the study were necropsied.
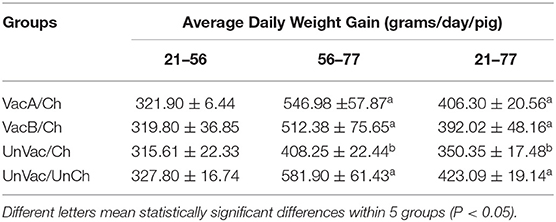
Average Daily Weight Gain
The live weight of each pig was measured at −35 dpc (21 days old), 0 dpc (56 days old), and 21 dpc (77 days old). Average daily weight gain (ADWG; grams/pig/day) was analyzed from the collected data over two time periods: (i) between 21 and 56 days of age and (ii) between 56 and 77 days of age. ADWG during these two production stages was then calculated as the difference between the starting and final weight divided by the duration of the stage. Data for dead or removed pigs were included in the calculation.
Quantification of M. hyopneumoniae in Nasal Swabs
A commercial kit (QIAamp DNA Mini Kit, QIAGEN, Valencia, California, USA) was used to extract DNA from nasal swabs prior to quantification of M. hyopneumoniae genomic DNA copy numbers by real-time PCR (20).
Quantification of PCV2d DNA in Blood
A commercial kit (QIAamp DNA Mini Kit, QIAGEN) was used to extract DNA from serum samples prior to quantification of PCV2 genomic DNA copy numbers by use of real-time PCR (21).
Serology
All collected serum samples were tested using the commercially available M. hyopneumoniae (M. hyo. Ab test, IDEXX Laboratories Inc.) and PCV2 (SERELISA PCV2 Ab Mono Blocking, Synbiotics) ELISA kits in accordance with the manufacturer's instructions. Serum samples were considered positive for M. hyopneumoniae antibody if the sample-to-positive (S/P) ratio was ≥0.4, while serum samples were considered to be positive for anti-PCV2 antibody if the reciprocal ELISA titer was >350.
Enzyme-Linked Immunospot (ELISPOT) Assay
The M. hyopneumoniae and PCV2d challenge strains were used in the in vitro stimulation of peripheral blood mononuclear cells (PBMC) so that the numbers of M. hyopneumoniae- and PCV2d-specific interferon-γ secreting cells (IFN-γ-SC) could be quantified (21, 22). All results were expressed as the numbers of IFN-γ-SC per million PBMC. The frequency of M. hyopneumoniae- and PCV2d-specific IFN-γ-SC was considered to be positive if the number of M. hyopneumoniae- and PCV2d-specific IFN-γ-SC was >20 cells/106 PBMC.
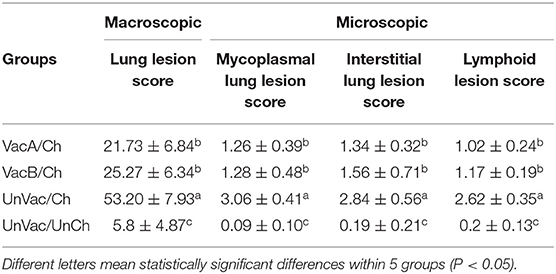
Pathology
Macroscopic lung lesion severity was scored to estimate the percentage of the lung affected by pneumonia (23).
Two veterinary pathologists (Chae and one graduate student) were blinded while examing lung and lymphoid tissue sections. Mycoplasmal pneumonia lesions were scored (0–6) based on the severity of peribronchiolar and perivascular lymphoid tissue hyperplasia (24). Interstitial pneumonia lesions were scored (0–6) based on the severity of interstitial pneumonia (19). PCV2-associated lymphoid lesions were scored (0–5) based on the severity of lymphoid depletion and granulomatous inflammation (10, 25).
Statistical Analysis
Real-time PCR data were transformed to log10 values prior to statistical analysis. Data were tested for normal distribution using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) was used to examine whether there were statistically significant differences among the four groups at each defined time point. Test results showing a statistical significance from one-way ANOVA were then subjected to a post-hoc test for a pairwise comparison with Tukey's adjustment. If the normality assumption was not met, the Kruskal-Wallis test was additionally performed. Any results showing statistical significance from the Kruskal Wallis test were then subjected to the Mann-Whitney test with Tukey's adjustment to compare the differences among the groups. A value of P < 0.05 was considered to be significant.
Results
Clinical Observation
In UnVac/Ch group pigs exhibited mild-to-moderate respiratory disease that was characterized by lethargy, coughing, and occasionally sneezing between 7 and 14 dpc. Moderate-to-severe respiratory disease that was characterized by coughing, depression, and pronounced abdominal breathing was also observed the same timeperiod. At 21 dpc, symptoms in the UnVaqc/Ch group pigs regressed to mild-to-moderate respiratory disease. Pigs in VacA/Ch and VacB/Ch groups showed minimal respiratory diseases that was characterized by occisionally coughing and sneezing. No respiratory diseases were observed in pigs from UnVac/UnCh group. The mean scores for respiratory disease from 0 to 21 dpc were signficantly higher (P < 0.05) in pigs from the UnVac/Ch group when compared with the VacA/Ch, VacB/Ch, and UnVac/UnCh groups (Figure 1).
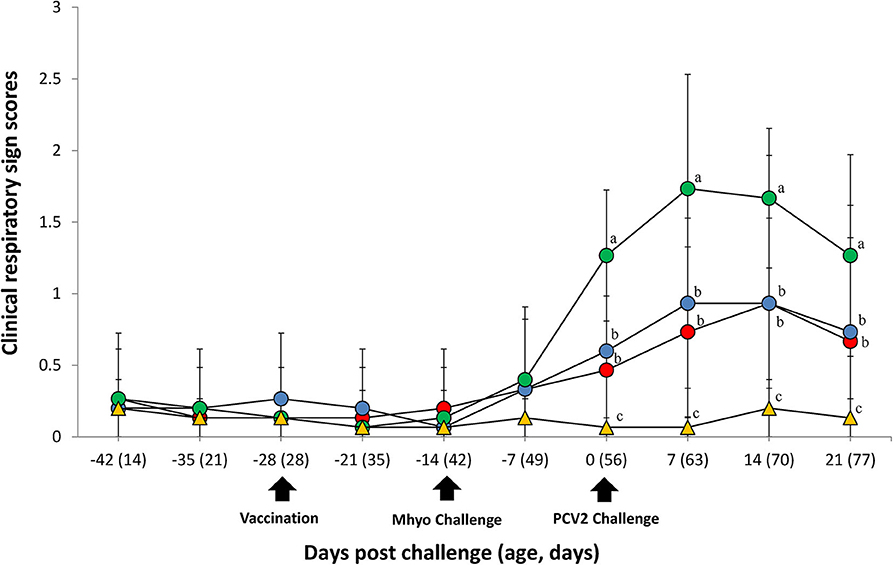
Figure 1. Clinical resipiratory sign. Clinical respiratory sign scores in the different groups: VacA/Ch (•), VacB/Ch (•), UnVac/Ch (•), and UnVac/UnCh (▴). Different letters within a sampling point mean statistically significant differences (P < 0.05). Variation is expressed as the standard deviation. Different letters within a sampling point mean statistically significant differences (P < 0.05).
Average Daily Weight Gain
All 4 groups were similar in average body weights at the start of the study (21 days of age). Pigs from the VacA/Ch, VacB/Ch, and UnVac/UnCh groups had significantly higher (P < 0.05) ADWG between 56 and 77 days of age when compared with pigs in the UnVac/Ch group. The overall ADWG results (from 21 to 77 days old) of pigs from the VacA/Ch, VacB/Ch, and UnVac/UnCh groups was significantly higher (P < 0.05) when compared with in the UnVac/Ch group. A significant difference was not calculated in the ADWG among the VacA/Ch, VacB/Ch, and UnVac/UnCh groups (Table 2).
Genomic Quantification of M. hyopneumoniae and PCV2
Prior to challenge, zero genomic copies of M. hyopneumoniae and PCV2 were detected in any of the pigs within the study. For genomic quantification of M. hyopneumoniae, pigs in the VacA/Ch group had significantly less (P < 0.05) M. hyopneumoniae genomic copies in their nasal swabs at 7 dpc when compared with the UnVac/Ch group. Pigs in the VacA/Ch and VacB/Ch groups had significantly less (P < 0.05) M. hyopneumoniae genomic copies in their nasal swabs at 14 and 21 dpc when compared with the UnVac/Ch group (Figure 2A).
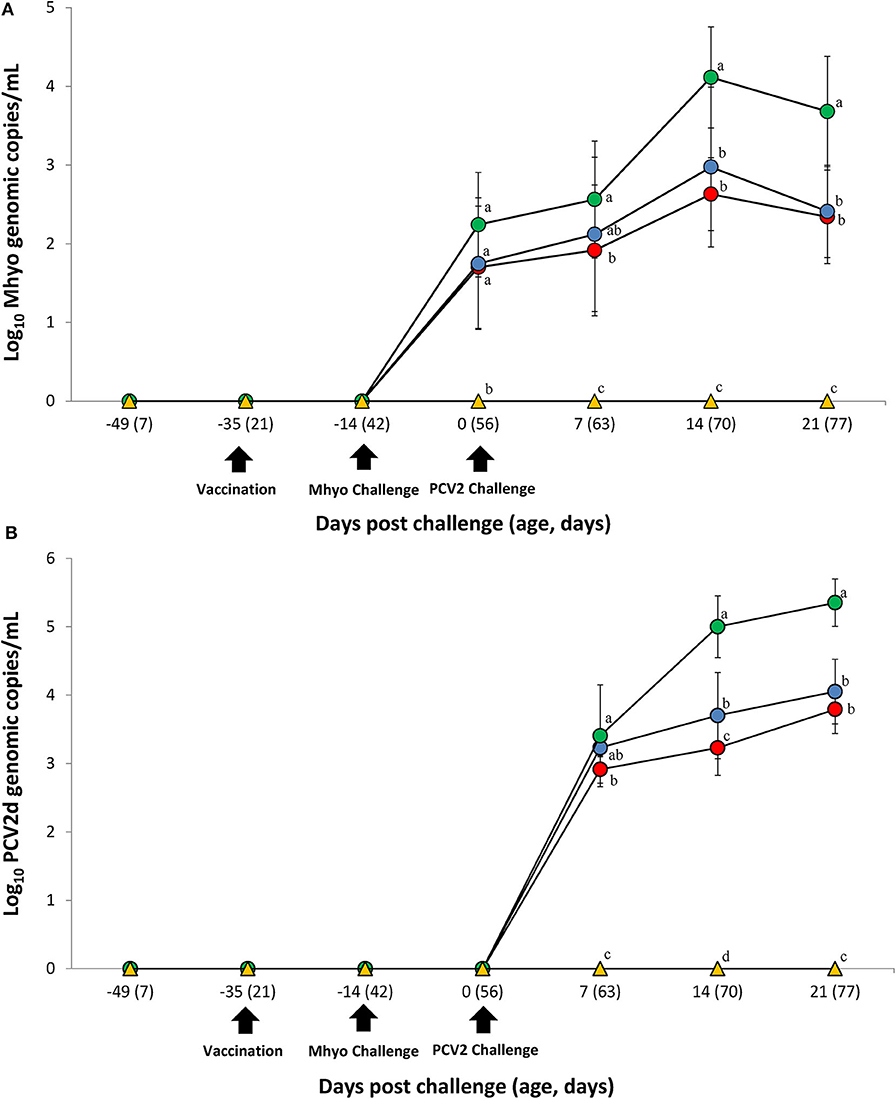
Figure 2. Genomic quantification. (A) Mean values of the genomic copy number of Mycoplasma hyopneumoniae (Mhyo) DNA in nasal swabs. (B) Mean values of the genomic copy number of porcine circoviorus type 2d (PCV2d) DNA in serum in the different groups: VacA/Ch (•), VacB/Ch (•), UnVac/Ch (•), and UnVac/UnCh (▴). Different letters within a sampling point mean statistically significant differences (P < 0.05). Variation is expressed as the standard deviation. Different letters within a sampling point mean statistically significant differences (P < 0.05).
For genomic quantification of PCV2, pigs in the VacA/Ch group had a significantly lower (P < 0.05) number of genomic copies of PCV2 in their blood at 7 dpc when compared with the UnVac/Ch group. Pigs in the VacA/Ch and VacB/Ch groups had a significantly lower (P < 0.05) number of genomic copies of PCV2 in their blood at 14 and 21 dpc when compared with the UnVac/Ch group. Pigs in the VacA/Ch group had a significantly lower (P < 0.05) number of genomic copies of PCV2 in their blood at 14 dpc when compared with the VacB/Ch group. No genomic copies of M. hyopneumoniae or PCV2 were detected in any of the pigs from the UnVac/UnCh group (Figure 2B).
Immune Responses Against M. hyopneumoniae
ELISA was used to assess pig antibody responses against M. hyopneumoniae. Pigs in the VacA/Ch and VacB/Ch groups had significantly higher (P < 0.05) M. hyopneumoniae ELISA S/P ratios between −14 and 21 dpc when compared with the UnVac/Ch groups. Antibodies against M. hyopneumoniae were not detected in any of the pigs from the UnVac/UnCh group (Figure 3A).
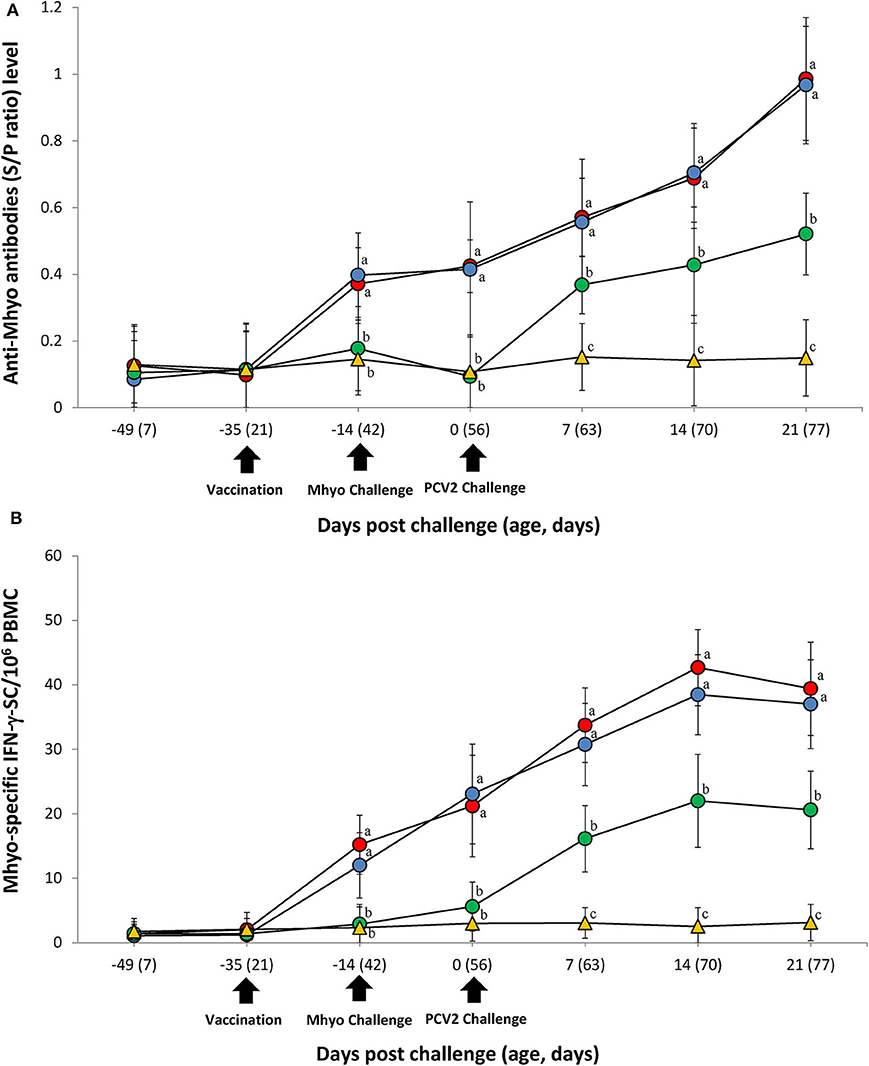
Figure 3. Immune responses against Mycoplasma hyopneumoniae (Mhyo). (A) Mean values of the Mhyo ELISA antibodies. (B) Frequency of Mhyo-specific interferon-γ secreting cells (IFN-γ-SC)/106 peripheral blood mononuclear cells (PBMC) in the different groups: VacA/Ch (•), VacB/Ch (•), UnVac/Ch (•), and UnVac/UnCh (▴). Variation is expressed as the standard deviation. Different letters within a sampling point mean statistically significant differences (P < 0.05).
The number of M. hyopneumoniae-specific IFN-γ-SC quantified in the PBMC of individual pigs was used to categorize T cell response. Pigs from the VacA/Ch and VacB/Ch groups had a significantly higher (P < 0.05) number of M. hyopneumoniae-specific IFN-γ-SC in their PBMC between −14 and 21 dpc when compared with pigs from the UnVac/Ch group. The mean numbers of M. hyopneumoniae-specific IFN-γ-SC in the UnVac/UnCh group remained at basal levels (<20 cells/106 PBMC) throughout the study (Figure 3B).
Immune Responses Against Porcine Circovirus Type 2
ELISA was used to assess pig antibody responses PCV2. Pigs in the VacA/Ch and VacB/Ch groups had significantly higher (P < 0.05) PCV2 ELISA titers between −14 and 21 dpc when compared with those in the UnVac/Ch groups. Antibodies against PCV2 were not detected in any of the pigs from the UnVac/UnCh group (Figure 4A).
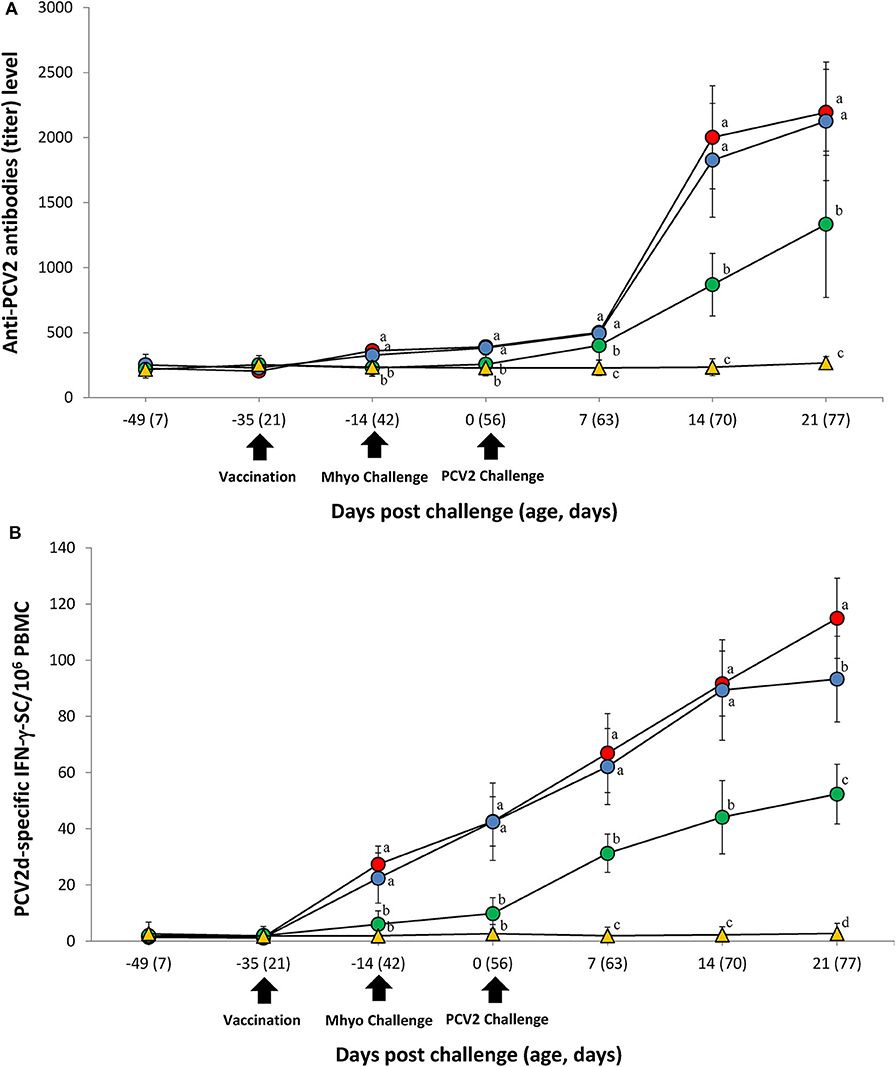
Figure 4. Immune responses against porcine circovirus type 2 (PCV2). (A) Mean values of the PCV2 ELISA antibodies. (B) Frequency of PCV2d-specific interferon-γ secreting cells (IFN-γ-SC)/106 peripheral blood mononuclear cells (PBMC) in the different groups: VacA/Ch (•), VacB/Ch (•), UnVac/Ch (•), and UnVac/UnCh (▴). Variation is expressed as the standard deviation. Different letters within a sampling point mean statistically significant differences (P < 0.05).
Pigs from the VacA/Ch and VacB/Ch groups had a significantly higher (P < 0.05) number of PCV2d-specific IFN-γ-SC in their PBMC between −14 and 21 dpc when compared with pigs in the UnVac/Ch group. Pigs from the VacA/Ch group had significantly higher (P < 0.05) numbers of PCV2d-specific IFN-γ-SC in their PBMC at 21 dpc when compared with pigs in the VacB/Ch group. The mean numbers of PCV2d-specific IFN-γ-SC in the UnVac/UnCh group remained at basal levels (<20 cells/106 PBMC) throughout the study (Figure 4B).
Pathology
Pigs in the VacA/Ch and VacB/Ch groups had significantly lower (P < 0.05) macroscopic lung lesion scores, microscopic mycoplasmal and interstitial lung lesion scores, and microscopic lymphoid lesions at 21 dpc when compared with pigs from the UnVac/Ch group. Macroscopic and microscopic lung lesions, and micriscopic lymphoid lesions were not observed in pigs from the UnVac/UnCh group (Table 3).
Discussion
Both presentations of the evaluated vaccines (a combination of two monovalent vaccines mixed into a combo prior to vaccination, or a ready-to-use bivalent vaccine) proved efficacious in the protection of pigs against M. hyopneumoniae and PCV2d challenge. The study included a successful challenge infection as all infected pigs tested positive for M. hyopneumoniae and PCV2d by PCR testing of nasal swabs and blood, respectively. The present data demonstrated that the two most prominent and costly diseases, PCVAD and enzootic pneumonia, can be prevented with either form of a combination vaccine, two monovalents which are mixed onsite prior to vaccination (a combination of two monovalent vaccines mixed into a combo prior to vaccination) or a ready-to-use vaccine. Growth performance was the most critical parameter evaluated throughout the study and should be given preccedece when evaluating the efficacy of a vaccine (26). Vaccination and challenge by either method resulted in significant growth performance improvement when compared with the unvaccinated-challenged group. Although ADWG was numerically higher in the mixable combo-vaccinated group than in the ready-to-use bivalent-vaccinated group, it was not determined as statistically significant.
Field conditions were mimicked through the vaccination-infection model design of this study. Analysis of diagnostic cases and serological surveyance under Korean field conditions (C. Chae, personal observation) has concluded that pigs are generally prone to M. hyopneumoniae infection at 5–7 weeks of age and to PCV2 infection at 7–9 weeks of age, which eventually leads to a PRDC outbreak at 11–16 weeks of age. These natural-occurring infection timeframes were kept in mind when designing this study, which is why pigs were inoculated with M. hyopneumoniae at 6 weeks of age followed by PCV2 inoculation at 8 weeks of age. In this sequential infection model, M. hyopneumoniae infection followed by PCV2 reproduced PCVAD experimentally, mimicking natural cases in the field as described in the previous study (27). Vaccination of pigs with PCV2 and M. hyopneumoniae at 3 weeks of age provided statistically significant protection against M. hyopneumoniae at 6 weeks of age and against PCV2 at 8 weeks of age.
Large opportunities still exist to develop a better understooding of protective immunity against M. hyopneumoniae. As a non-invasive pathogen that can induce pneumonia, cell-mediated immune response must play a significant immulogical role. A consistent correlation between the induction of cell-mediated immunity and the severity of pneumonic lesions has been demonstrated (28, 29). A significant difference was not found between the two sets of vaccines used in this study, in terms of elicited cell-mediated immune response and protective effect against M. hyopneumoniae challenge. The strain of M. hyopneumponiae was identical in both sets of vaccines (VacA/Ch and VacB/Ch groups) but the vaccine adjuvants varied (30). Although adjuvant formulation in particular is known to affect the immunogenicity and protective effect of inactivated whole-cell M. hyopneumoniae bacterins (31), an effect on efficacy against M. hyopneumoniae was not observed in the present study.
Both vaccines evaluated in this study were based on the PCV2a genotype. PCV2d viremia was still reduced in pigs from PCV2a-based vaccination and PCV2d-challenge when compared with unvaccinated and challenged pigs. Reduction of PCV2d Viremia is clinically meaningful as PCV2d is currently the predominant PCV2 genotype circulating within Asian pig herds (8). The reduction of PCV2 viremia is well-correlated with protection against PCV2 infection (32–34). Both types of vaccines elicited the PCV2d-specific IFN-γ-SC which is critical in reducing the amount of PCV2 viremia within pigs (35–37). PCV2d viremia and lymphoid lesion reduction is both attributed to the induction of protective cell-mediated imminuty. The two vaccines elicited different cell-mediated immune responses and levels of PCV2d viremia against PCV2d challenge. Although both vaccines used in this study have the same baculovirus system for the expression of the ORF2 subunit of PCV2, each vaccine contained a unique antigen preparation by use of the baculovirus expression system (38). This as well as differences in adjuvant formulation between the vaccines may be as the reasoning behind the differences in protective cell-mediated immunity and induction and reduction of both PCV2 viremia and lymphoid lesions. Further studies are needed to elucidate these differences between the two vaccines.
This comparative study under experimental conditions was the first evaluation between a combination of two monovalent vaccines mixed into a combo prior to vaccination and a ready-to-use bivalent vaccine of PCV2 and M. hyopneumoniae. Vaccination remains the most efficient and economical method to-date for the control of PCVAD and enzootic pneumonia. More than 70 percent of Korean pigs receive vaccines to protect against PCV2 and M. hyopneumoniae (http://www.kahpha.or.kr) as PCVAD and enzootic pneumonia remain the two most prominent and costly diseases that affect swine. An important advantage in using either a combination of two monovalent vaccines mixed into a combo prior to vaccination or a ready-to-use bivalent vaccine exists as either reduces the number of injections administered to the pig. The results of this study demonstrate a strategic method in controlling two important diseases for swine producers and practitioners.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Seoul national university Instituional Animal Care and Use Committee (SNU-190712-5).
Author Contributions
SY performance of the experimental trials, data analysis, and writing of the manuscript. TO, KP, and HC preparation of the inoculum and lab analysis. CC development of protocol, design of the study, review of the final manuscript, and approval for publication. All authors read and approved the final manuscript.
Funding
The author's research was supported by contract research funds (Grant No. 550-20190078) of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine and by the BK 21 Plus Program (Grant No. 5260-20150100) for Creative Veterinary Science Research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chae C. Porcine respiratory disease complex: interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet J. (2016) 212:1–6. doi: 10.1016/j.tvjl.2015.10.030
2. Thacker EL. Porcine respiratory disease complex what is and why does it remain a problem?. Pig J. (2001) 48:66–70.
3. Mankertz A, Persson F, Mankertz J, Blaess G, Buhk HJ. Mapping and characterization of the origin of DNA replication of porcine circovirus. J Virol. (1997) 71:2562–6. doi: 10.1128/JVI.71.3.2562-2566.1997
4. Dupont K, Nielsen EO, Baekbo P, Larsen LE. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case control study supports a shift in genotypes with time. Vet Microbiol. (2008) 128:56–64. doi: 10.1016/j.vetmic.2007.09.016
5. Wang F, Guo X, Ge X, Wang Z, Chen Y, Cha Z, et al. Genetic variation analysis of Chinese strains of porcine circovirus type 2. Virus Res. (2009) 145:151–6. doi: 10.1016/j.virusres.2009.05.015
6. Davies B, Wang X, Dvorak CMT, Marthaler D, Murtaugh MP. Diagnostic phylogenetics reveals a new porcine circovirus 2 cluster. Virus Res. (2016) 217:32–7. doi: 10.1016/j.virusres.2016.02.010
7. Bao F, Mi S, Luo Q, Guo H, Tu C, Zhu G, et al. Retrospective study of porcine circovirus type 2 infection reveals a novel genotype PCV2f. Transboud Emerg Dis. (2018) 65:432–40. doi: 10.1111/tbed.12721
8. Kwon T, Lee D-U, Yoo SJ, Je SH, Shin JY, Lyoo YS. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Res. (2017) 228:24–9. doi: 10.1016/j.virusres.2016.11.015
9. Xiao C-T, Harmon KM, Halbu PG, Opriessnig T. PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the U.S. during 2014-2016. Vet Microbiol. (2016) 197:72–7. doi: 10.1016/j.vetmic.2016.11.009
10. Chae C. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet J. (2004) 168:41–9. doi: 10.1016/S1090-0233(03)00182-5
11. Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. (2005) 169:326–36. doi: 10.1016/j.tvjl.2004.01.012
12. Segalés J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. (2012) 164:10–9. doi: 10.1016/j.virusres.2011.10.007
13. Thacker EL, Christopher Minion C. Mycoplasmosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th edn. Ames, IA: Wiley-Blackwell. (2012). p. 779–7.
14. Kim J, Chun H-K, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. (2003) 166:251–6. doi: 10.1016/S1090-0233(02)00257-5
15. Cohen J. Statistical Power Analysis for the Behavioral Science. 2nd ed. New York, NY: Routledge (1988). p. 273–406.
16. Van Reeth K, Nauwynck H, Pensaert M. A potential role for tumour necrosis factor-α in synergy between porcine respiratory coronavirus and bacterial lipopolysaccharide in the induction of respiratory disease in pigs. J Med Microbiol. (2000) 49:613–20. doi: 10.1099/0022-1317-49-7-613
17. Marchioro SB, Sacristan RDP, Michiels A, Haesebrouck F, Conceição F, Dellagostin O, et al. Immune responses of a chimeric protein vaccine containing Mycoplasma hyopneumoniae antigens and LTB against experimental M. hyopneumoniae infection in pigs. Vaccine. (2014) 32:4689–94. doi: 10.1016/j.vaccine.2014.05.072
18. Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, et al. 2000 report of the AVMA panel on euthanasia. J Am Vet Med Assoc. (2001) 218:669–96. doi: 10.2460/javma.2001.218.669
19. Halbur PG, Paul PS, Frey M, Landgraf J, Eernisse K, Meng XJ, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. (1995) 32:648–60. doi: 10.1177/030098589503200606
20. Dubosson CR, Conzelmann C, Miserez R, Boerlin P, Frey J, Zimmermann W, et al. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet Microbiol. (2004) 102:55–65. doi: 10.1016/j.vetmic.2004.05.007
21. Jeong J, Park C, Choi K, Chae C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet Microbiol. (2015) 177:43–52. doi: 10.1016/j.vetmic.2015.02.027
22. Jeong J, Kang I, Kim S, Park KH, Park C, Chae C. Comparison of 3 vaccination strategies against porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, and porcine circovirus type 2 on 3 pathogen challenge model. Can J Vet Res. (2018) 82:39–47.
23. Garcia-Morante B, Segalés J, Farile L, Perez de Rozas A, Maiti H, Coll T, et al. Assessment of Mycoplasma hyopneumoniae-induced pneumonia using different lung lesion scoring system: a comparative review. J Comp Pathol. (2016) 154:125–34. doi: 10.1016/j.jcpa.2015.11.003
24. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. (1999) 37:620–7. doi: 10.1128/JCM.37.3.620-627.1999
25. Kim J, Chae C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J Comp Pathol. (2004) 131:121–6. doi: 10.1016/j.jcpa.2004.02.001
26. Chae C. Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet J. (2012) 194:151–7. doi: 10.1016/j.tvjl.2012.06.031
27. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng X-J, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. (2004) 41:624–40. doi: 10.1354/vp.41-6-624
28. Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust Vet J. (1997) 75:504–11. doi: 10.1111/j.1751-0813.1997.tb14383.x
29. Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am J Vet Res. (2000) 61:1384–9. doi: 10.2460/ajvr.2000.61.1384
30. Tao Y, Shu J, Chen J, Wu Y, He Y. A concise review of vaccines against Mycoplasma hyopneumoniae. Res Vet Sci. (2019)123:144–52. doi: 10.1016/j.rvsc.2019.01.007
31. Galliher-Beckley A, Pappan LK, Madera R, Burakova Y, Waters A, Nickles M, et al. Characterization of a novel oil-in-water emusion adjuvant for swine influenza virus and Mycoplasma hyopneumoniae vaccines. Vaccine. (2015) 33:2903–8. doi: 10.1016/j.vaccine.2015.04.065
32. Seo HW, Han K, Oh Y, Park C, Chae C. Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine. (2012) 30:6671–7. doi: 10.1016/j.vaccine.2012.08.065
33. Fort M, Sibila M, Perez-Martin E, Nofrarias M, Mateu E, Segalés J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicit cell-mediated immunity and significantly reduced PCV2 viremia in an experimental model. Vaccine. (2009) 27:4031–7. doi: 10.1016/j.vaccine.2009.04.028
34. Martelli P, Ferrari L, Morganti M, Angelis DE, Bonilauri P, Guazzetti S, et al. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet Microbiol. (2011) 149:339–51. doi: 10.1016/j.vetmic.2010.12.008
35. Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. (2008) 26:1063–71. doi: 10.1016/j.vaccine.2007.12.019
36. Fort M, Sibila M, Nofrarias M, Perez-Martin E, Olvera A, Mateu E, et al. Evaluation of cell-mediated immune responses against porcine circovirus type 2 (PCV2) Cap and Rep proteins after vaccination with a commercial PCV2 sub-unit vaccine. Vet Immunol Immunopathol. (2012) 150:128–32. doi: 10.1016/j.vetimm.2012.09.001
37. Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2-3-month post vaccination. Vaccine. (2009) 27:1002–7. doi: 10.1016/j.vaccine.2008.11.105
Keywords: Mycoplasma hyopneumoniae, porcine circovirus type 2, porcine respiratory disease complex, vaccination, swine, bivlent vaccine, dual challenge
Citation: Yang S, Oh T, Park KH, Cho H and Chae C (2020) A Dual Swine Challenge With Porcine Circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae Used to Compare a Combination of Mixable Monovalent PCV2 and Monovalent M. hyopneumoniae Vaccines With a Ready-to Use PCV2 and M. hyopneumoniae Bivalent Vaccine. Front. Vet. Sci. 7:579. doi: 10.3389/fvets.2020.00579
Received: 02 June 2020; Accepted: 20 July 2020;
Published: 02 September 2020.
Edited by:
Lester J. Perez, University of Illinois at Urbana–Champaign, United StatesReviewed by:
Lorenzo Fraile, Universitat de Lleida, SpainPatrick Gerard Halbur, Iowa State University, United States
Copyright © 2020 Yang, Oh, Park, Cho and Chae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanhee Chae, swine@snu.ac.kr
 Siyeon Yang
Siyeon Yang  Chanhee Chae
Chanhee Chae