Abstract—
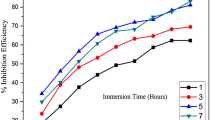
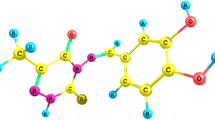
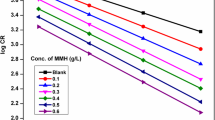
The inhibiting effect of the expired meloxicam on the corrosion of mild steel (MS) in 1 M HCl was tested by the weight loss, electrochemical frequency modulation, potentiodynamic polarization (PP) and electrochemical impedance spectroscopy techniques. The PP curves showed that this drug acts as mixed type inhibitor. This drug was adsorbed chemically on the MS surface following the Temkin adsorption isotherm. Some thermodynamic parameters were computed and discussed. The results indicated that the protection efficiency increases by raising both the doses of the inhibitor and the temperature of the medium. The morphology of the MS surface was analyzed by scanning electron microscopy, atomic force microscopy, and Fourier transform infrared spectroscopy. All of the obtained results from different techniques are similar.












Similar content being viewed by others
REFERENCES
Musa, A.Y., Khadom, A.A., and Kadhum, A.H., J. Taiwan Inst. Chem. Eng., 2010, vol. 41, pp. 126–128.
Ameer, M.A. and Fekry, A.M., Int. J. Hydrogen Energy, 2010, vol. 35, pp. 11387–11396.
Loto, R.T., Loto, C.A., and Popoola, A.P.I., J. Mater. Environ. Sci., 2012, vol. 3, pp. 885–894.
Ladha, D.G., Naik, U.J., and Shah, N.K., J. Mater. Environ. Sci., 2013, vol. 4, pp. 701–708.
Hajjaji, N., Ricco, I., Srhiri, A., Lattes, et al., Corrosion, 1993, vol. 49, pp. 326–334.
Elachouri, M., Hajji, M.S., Salem, M., Kertit, S., et al., Corros. Sci., 1995, vol. 37, pp. 381–389.
Luo, H., Guan, Y.C., and Han, K.N., Corrosion, 1998, vol. 54, pp. 619–627.
Migahed, M.A., Azzam, E.M.S., and Al-Sabagh, A.M., Mater. Chem. Phys., 2004, vol. 85, pp. 273–279.
Osman, M.M., Omar, A.M., and Al-Sabagh, A.M., Mater. Chem. Phys., 1997, vol. 50, pp. 271–274.
Zucchi, F., Trabanelli, G., and Brunoro, G., Corros. Sci., 1992, vol. 33, pp. 1135–1139.
Villamil, R.F.V., Corio, P., Rubim, J.C., and Siliva Agostinho M.L., J. Electroanal. Chem., 1999, vol. 472, pp. 112–119.
Zhao, T.P. and Mu, G.N., Corros. Sci., 1999, vol. 41, pp. 1937–1944.
Abd El Rehim, S.S., Hassan, H., and Amin, M.A., Mater. Chem. Phys., 2001, vol. 70, pp. 64–72.
Abd El Rehim, S.S., Hassan, H., and Amin, M.A., Mater. Chem. Phys., 2003, vol. 78, pp. 337–348.
Guo, R., Liu, T., and Wei, X., Colloids Surf., 2002, vol. 209, pp. 37–45.
Branzoi, V., Golgovici, F., and Branzoi, F., Mater. Chem. Phys., 2002, vol. 78, pp. 122–131.
Bentiss, T.F. and Lagrenee, M., Corros. Sci., 2000, vol. 42, pp. 127–146.
Brett C.M.A., Gomes I.A.R., and Martins J.P.S., Corros. Sci., 1994, vol. 36, pp. 915–923.
Elachouri, M. Hajji, M., Salem, M., Kertit, S., et al., Corrosion, 1996, vol. 52, pp. 103–108.
Algaber, A.S., El-Nemma, E.M., and Saleh, M.M., Mater. Chem. Phys., 2004, vol. 86, pp. 26–32.
Oukhrib, R., El Ibrahimi, B., Bourzi, H., El Mouaden, K., et al., J. Mater. Environ. Sci., 2017, vol. 8, no. 1, pp. 195–208.
Al-Azzawi, A.M. and Hammud, K.K., Int. J. Res. Pharm. Chem., 2016, vol. 6, no. 3, pp. 391–402.
El Ouasif, L., Merimi, I., Zarrok, H., El Ghoul, M., et al., J. Mater. Environ. Sci., 2016, vol. 7, no. 8, pp. 2718–2730.
Sani, U.M. and Sman, U.U., Int. J. Risk Contingency Manage., 2016, vol. 3, no. 3, pp. 30–37.
Kolo, A.M., Sani, U.M., Kutama, U., and Usman, U., Pharm. Chem. J., 2016, vol. 3, no. 1, pp. 109–119.
Ameh, P.O. and Sani, U.M., J. Heterocyclics, 2015, vol. 101, pp. 2–6.
Kushwah, R. and Pathak, R.K., J. Emerging Technol. Adv. Eng., 2014, vol. 4, no. 7, pp. 880–884.
Fouda, A.S., EL-Haddad, M.N., and Abdallah, Y.M., Int. J. Innovative Res. Sci.,Eng. Technol., 2013, vol. 2, no. 12, pp. 7073–7085.
Ofoegbu, S.U. and Ofoegbu, P.U., J. Eng. Appl. Sci., 2012, vol. 7, no. 3, pp. 272–276.
Mu, G.N., Zhao, T.P., Liu, M., and Gu, T., Corrosion, 1996, vol. 52, pp. 853–856.
Lipkowski, J. and Ross, P.N., Adsorption of Molecules at Metal Electrodes, New York: Wiley, 1992.
Da Costa, S.L.F.A. and Agostinho, S.M.L., Corrosion, 1989, vol. 45, pp. 472–477.
Aljourani, J., Raeissi, K., and Golozar, M.A., Corros. Sci., 2009, vol. 51, pp. 1836–1843.
Ivanov, E.S., Ingibitory korrozii metallov v kislykh sredakh (Inhibitors for Metal Corrosion in Acid Media), Moscow: Metallurgiya, 1986.
Lebrini, M., Bentiss, F., Vezin, H., and Lagrenee, M., Corros. Sci., 2006, vol. 48, pp. 1279–1291.
Hour, T.P. and Holliday, R.D., J. Appl. Chem., 1953, vol. 3, pp. 502–513.
Riggs, L.O. and Hurd, T.J., Corrosion, 1967, vol. 23, pp. 252–260.
Schmid, G.M. and Huang, H.J., Corros. Sci., 1980, vol. 20, pp. 1041–1057.
Kus, E. and Mansfeld, F., Corros. Sci., 2006, vol. 48, pp. 965–979.
Tang, L, Li, X, Si, Y., Mu, G., et al., Mater. Chem. Phys., 2006, vol. 95, pp. 29–38.
Caigman, G.A., Metcalf, S.K., and Holt, E.M., J. Chem. Cryst., 2000, vol. 30, pp. 415–422.
Trabanelli, G., Montecelli, C., Grassi, V., and Frignani, A., Cem. Concr. Res., 2005, vol. 35, pp. 1804–1813.
McCafferty E. and Hackerman N., J. Electrochem. Soc., 1972, vol. 119, pp. 146–154.
Ma, H., Chen, S., Niu, L., Zhao, S., et al., J. Appl. Electrochem., 2002, vol. 32, pp. 65–72.
Fouda A.S., Ibrahim, H., and Atef, M., Results Phys., 2017, vol. 7, pp. 3408–3418.
Patmore, H., Jebreel, A., Uppal, S., Raine, C.H., et al., Am. J. Otolaryngol., 2010, vol. 31, no. 5, pp. 376–380.
Ridder, G.J., Breunig, C., Kaminsky, J., and Pfeiffer, J., Eur. Arch. Otorhinolaryngol., 2015, vol. 272, no. 5, pp. 1269–1276.
Kraus, D.H., Rehm, S.J., and Kinney, S.E., Laryngoscope, 1988, vol. 98, no. 9, pp. 934–939.
Sharma, P., Agarwal, K.K., Kumar, S., Singh, H., et al., Jpn. J. Radiol., 2013, vol. 31, no. 2, pp. 81–88.
Stokkel, M.P., Boot, C.N., and van Eck-Smit, B.L., Laryngoscope, 1996, vol. 106, no. 3, pp. 338–340.
Clark, M.P., Pretorius, P.M., Byren, I., and Milford, C.A., Skull Base, 2009, vol. 19, no. 4, pp. 247–254.
Okpala, N.C., Siraj, Q.H., Nilssen, E., and Pringle, M., J. Laryngol. Otol., 2005, vol. 119, no. 1, pp. 71–75.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare to have no conflict of interest.
About this article
Cite this article
Fouda, A.S., El-Dossoki, F.I., El-Hossiany, A. et al. Adsorption and Anticorrosion Behavior of Expired Meloxicam on Mild Steel in Hydrochloric Acid Solution. Surf. Engin. Appl.Electrochem. 56, 491–500 (2020). https://doi.org/10.3103/S1068375520040055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375520040055