Abstract
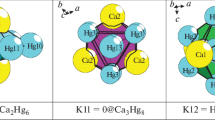
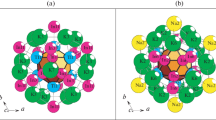
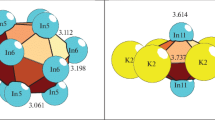
The combinatorial-topological analysis and simulation of self-assembly of the Na96Hg36-hR132 (space group R-3c, a = b = 9.228 Å, c = 52.6380 Å, V = 3881.91 Å3) and Na12Hg8-tP20 (space group P42/mnm, a = b = 8.520, c = 7.800 Å, V = 566.2 Å3) crystal structures are conducted by computer-based methods (ToposPro program package). Polyhedral cluster precursors K11 = 0@11(Na8Hg3) and K9 = Hg@Na8 are first determined for the intermetallic compound Na96Hg36-hR132, while polyhedral cluster precursors K5 = 0@Na3Hg2 are determined for the intermetallic compound Na12Hg8-tP20. The symmetry and topology code of the self-assembly processes of the 3D structure Na96Hg36-hR132 from the nanocluster precursors K11 and K9, and of the 3D structure Na12Hg8-tP20 from the K5 clusters are reconstructed as the primary chain \(S_{{\text{3}}}^{1}\) → layer \(S_{{\text{3}}}^{2}\)→ frame \(S_{{\text{3}}}^{3}.\) The structural analysis of all known intermetallic compounds is conducted, and numerous examples of the assembly of their structures from the K5, K9, and K11 clusters are found.







Similar content being viewed by others
REFERENCES
Hoch, C. and Simon, A., Na11Hg52: Complexity in a polar metal, Angew. Chem., Int. Ed. Engl., 2012, vol. 51, no. 13, pp. 3262–3265.
Nielson, J.W. and Baenziger, N.C., The crystal structrues of NaHg2, NaHg and Na3Hg2, Acta Crystallogr., 1954, vol. 7, pp. 277–282.
Deiseroth, H.J., Stupperich, A., Pankaluoto, R., and Christensen, N.E., A variant of the cesium chloride structure: Structural relations and electronic structure, Z. Anorg. Allg. Chem., 1991, vol. 597, pp. 41–50.
Tkachuk, A.V. and Mar, A., Redetermination of Na3Hg2, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, vol. 62, pp. i129–i130.
Deiseroth, H.J. and Toelstede, D., Na8Hg3: An alkali metal rich amalgam with isolated mercury anions?, Z. Anorg. Allg. Chem., 1990, vol. 587, pp. 103–109.
Deiseroth, H.J. and Rochnia, M., Single-crystal studies on the temperature dependence of the crystal structure of alpha Na3Hg, Z. Anorg. Allg. Chem., 1994, vol. 620, pp. 1736–1740.
Deiseroth, H.J. and Rochnia, M., β-Na3Hg: A solid with molten sodium partial structure in the temperature range of 36–60°C, Angew. Chem., 1993, vol. 105, pp. 1556–1558.
Inorganic Crystal Structure Database (ICSD), Fachinformationszentrum, Karlsruhe, Germany, and US Natl. Inst. of Standard and Technology (NIST), USA.
Bruzzone, G. and Merlo, F., The calcium-mercury system, J. Less-Common Met., 1973, vol. 32, pp. 237–241.
Tkachuk, A.V. and Mar, A., Alkaline-earth metal mercury intermetallics A(11 – x) Hg(54 + x) (A = Ca, Sr), Inorg. Chem., 2008, vol. 47, no. 4, pp. 1313–1318.
Bobev, S. and Sevov, S.C., Synthesis and characterization of the largest isolated clusters of Tin, (Sn12)–12, in (AE) Na10Sn12 (AE = Ca or Sr), Inorg. Chem., 2001, vol. 40, pp. 5361–5364.
Todorov, I. and Sevov, S.C., In search of benzene-like \({\text{Sn}}_{6}^{{6 - }}\): Synthesis of Na4CaSn6 with interconnected cyclohexane-like \({\text{Sn}}_{6}^{{6 - }}\), Inorg. Chem., 2006, vol. 45, pp. 4478–4483.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Modeling self-organization processes in crystal-forming systems: Suprapolyedic Na18Hg157 precursor clusters for the self-assembly of the Na99Hg468-hP567 crystal structure, Glass Phys. Chem., 2019, vol. 45, no. 6, pp. 399–404.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Cluster self-organization of intermetallic systems: New two-layer cluster-precursor K46 = 0@ 8(Ca2Hg6)@38(Hg6 + CaHg6)2(Ca6Hg6) for self-assembly of the crystal structure of Ca11Hg54-hP65, Glass Phys. Chem., 2020, vol. 46, no. 1, pp. 1–5.
Marsh, R.E., The centrosymmetric-noncentrosymmetric ambiguity: Some more examples, Acta Crystallogr., Sect. A: Found. Crystallogr., 1994, vol. 50, pp. 450–455.
Range, K.J. and Büchler, H., High-pressure synthesis and crystal structure of Al3Au8, J. Less-Common Met., 1989, vol. 154, pp. 251–260.
Cirafici, S. and Fornasini, M.L., Crystal structures of Yb2Tl, Yb8Tl3 and Yb8In3, J. Less-Common Met., 1989, vol. 154, pp. 79–88.
Gaebler, F. and Niewa, R., Polymorphism of Eu8In3 and the solid solution (Ca(x) Eu(1 – x))8 In3, Z. Anorg. Allg. Chem., 2010, vol. 636, pp. 1803–1809.
Blatov, V.A., Shevchenko, A.P., and Proserpio, D.M., Applied topological analysis of crystal structures with the program package ToposPro, Cryst. Growth Des., 2014, vol. 14, pp. 3576–3585.
Ilyushin, G.D., Modelirovanie protsessov samoorganizatsii v kristalloobrazuyushchikh sistemakh (Modeling of Self-Organization Processes in Crystal-Forming Systems), Moscow: Editorial URSS, 2003.
Ilyushin, G.D., Symmetry and topology code of the cluster self-assembly of intermetallic compounds A[16]2B[12]4 of the Friauf families Mg2Cu4 and Mg2Zn4, Crystallogr. Rep., 2018, vol. 63, pp. 543–552.
Ilyushin, G.D., Crystal chemistry of lithium intermetallic compounds: A survey, Russ. J. Inorg. Chem., 2018, vol. 63, no. 14, pp. 1786–1799.
Ilyushin, G.D., Modeling of the self-organization processes in crystal-forming systems. Tetrahedral metal clusters and the self-assembly of crystal structures of intermetallic compounds, Crystallogr. Rep., 2017, vol. 62, pp. 670–683.
Shevchenko, V.Ya., Blatov, V.A., and Ilyushin, G.D., Cluster self-organization of intermetallic systems. New cluster presursor (InNa5)(AuAu5) and primary chain with the 5m symmetry for the self-assembly of the Na32Au44In24-oP100 crystal structure, Glass Phys. Chem., 2019, vol. 45, no. 4, pp. 245–250.
Ilyushin, G.D., Cluster self-organization of intermetallic systems: 124-Atom cluster 0@12(Ga12)@32(Li20Ga12)@80(Li4Na16 Ga60) and 44-atom cluster 0@12(Ga12)@32(Li2Na18Ga12) for the self-assembly of Li48Na80Ga332-oF920 crystal structure, Crystallogr. Rep., 1997, vol. 39, no. 8, pp. 857–861.
Shevchenko, V.Ya., Medrish, I.V., Ilyushin, G.D., and Blatov, V.A., From clusters to crystals: Scale chemistry of intermetallics, Struct. Chem., 2019, vol. 30, pp. 2015–2027.
Pankova, A.A., Akhmetshina, T.G., Blatov, V.A., and Proserpio, D.M., A collection of topological types of nanoclusters and its application to icosahedron-based intermetallics, Inorg. Chem., 2015, vol. 54, pp. 6616–6630.
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 19-02-00636) and Ministry of Science and Higher Education as part of a state order for the Federal Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Shevchenko, V.Y., Ilyushin, G.D., Medrish, I.V. et al. Cluster Self-Organization of Intermetallic Systems: Role of K5 = 0@5, K9 = 1@8 and K11 = 0@11 Clusters in the Self-Assembly of Crystal Structures. Glass Phys Chem 46, 277–284 (2020). https://doi.org/10.1134/S1087659620040112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659620040112