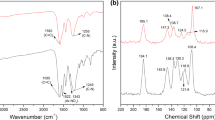
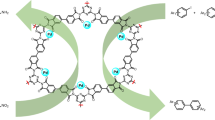
Abstract
Covalent organic frameworks (COFs) are emerging novel catalyst carrier. In this work, Pd/COF-LZU1 was one-pot prepared by p-phenylenediamine (PDA), triformylbenzene (TFB), and palladium acetate (Pd(OAc)2) under the catalysis of acetic acid (HAc). The effects of HAc concentration on the Pd/COF-LZU1 particle size, Pd loading, and catalytic activity in the reduction of 4-nitrophenol (4-NP) were investigated. It was found that, with the increased HAc concentration, the Pd/COF-LZU1 particle size reduced, and Pd loading and catalytic activity displayed maximum for 3 mol L−1 HAc. The catalytic reduction of 4-NP can be described by the pseudo-first-order kinetic model, and the rate constant is 20 times higher than that of palladium acetate. Pd/COF-LZU1 also showed excellent stability and reusability, demonstrating great potential in catalytic reduction reactions.

HAc displayed effects on particle size, Pd loading, and catalytic activity. Pd/COF-LZU1 prepared by one-pot method showed excellent catalytic performance.







Similar content being viewed by others
Abbreviations
- COFs:
-
Covalent organic frameworks
- PDA:
-
p-Phenylenediamine
- TFB:
-
Triformylbenzene
- Pd(OAc)2 :
-
Palladium acetate
- 4-NP:
-
4-Nitrophenol
- HAc:
-
Acetic acid
References
Bo L, Quan X, Wang X, Chen S (2008) Preparation and characteristics of carbon-supported platinum catalyst and its application in the removal of phenolic pollutants in aqueous solution by microwave-assisted catalytic oxidation. J Hazard Mater 157:179–186
Bódalo A, Gómez JL, Gómez M, León G, Hidalgo AM, Ruíz MA (2008) Phenol removal from water by hybrid processes: study of the membrane process step. Desalination 223:323–329
Bunck DN, Dichtel WR (2012) Internal functionalization of three-dimensional covalent organic frameworks. Angew Chem Int Ed 51:1885–1889
Dinesh et al (2018) Highly stable COF-supported Co/Co(OH)2 nanoparticles heterogeneous catalyst for reduction of nitrile/nitro compounds under mild conditions. Small. 14:e1801233
Ding SY, Wang W (2012) Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev 42:548–568
Ding SY, Gao J, Wang Q, Zhang Y, Song WG, Su CY, Wang W (2011) Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J Am Chem Soc 133:19816–19822
Ding S, Yang Y, Huang H, Liu H, Hou LA (2015) Effects of feed solution chemistry on low pressure reverse osmosis filtration of cesium and strontium. J Hazard Mater 294:27–34
Du X, Zhao C, Li X, Huang H, Wen Y, Zhang X, Li J (2017) Novel yolk-shell polymer/carbon@ Au nanocomposites by using dendrimer-like mesoporous silica nanoparticles as hard template. J Alloy Compd 700:83–91
Ebert S, Rieger P, Knackmuss H-J (1999) Function of coenzyme F420 in aerobic catabolism of 2,4, 6-trinitrophenol and 2,4-dinitrophenol by Nocardioides simplex FJ2-1A. J Bacteriol 181:2669–2674
Gao JK, Hou LA, Zhang GH, Gu P (2015) Facile functionalized of SBA-15 via a biomimetic coating and its application in efficient removal of uranium ions from aqueous solution. J Hazard Mater 286:325–333
Gatard S, Salmon L, Deraedt C, Ruiz J, Astruc D, Bouquillon S (2014) Palladium nanoparticles stabilized by glycodendrimers and their application in catalysis. Eur J Inorg Chem 26:4369–4375
Giannis A, Sandhoff K (2010) LiBH4(NaBH4)/Me3SiCl, an unusually strong and versatile reducing agent. Angew Chem. Int. Ed. 28:218–220
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J Catal 115:301–309
Jin Z, Xiao M, Bao Z, Wang P, Wang J (2012) A general approach to mesoporous metal oxide microspheres loaded with noble metal nanoparticles. Angew Chem Int Ed 124:6512–6516
Kalidindi SB, Oh H, Hirscher M, Esken D, Wiktor C, Turner S, van Tendeloo G, Fischer RA (2012) Metal@COFs: covalent organic frameworks as templates for Pd nanoparticles and hydrogen storage properties of Pd@COF-102 hybrid material. Chemistry. 18:10848–10856
Koyuncu H, Yıldız N, Salgın U, Köroğlu F, Calımlı A (2011) Adsorption of o-, m- and p-nitrophenols onto organically modified bentonites. J Hazard Mater 185:1332–1339
Kuppler RJ et al (2009) Potential applications of metal-organic frameworks. Coordination Chem Rev 253:3042–3066
Lemaire-Audoire S, Savignac M, Pourcelot G, Genêt JP, Bernard JM (1997) Chemoselective removal of allylic protecting groups using water-soluble Pd(OAc) 2/TPPTS catalyst. J Mol Catal A Chem 116:247–258
Li P, Zeng HC (2016) Immobilization of metal-organic framework nanocrystals for advanced design of supported nanocatalysts. ACS Appl Mater Interfaces 8:29551–29564
Lin JR, Gubaidullin AT, Mamedov VA, Tsuboi S (2002) Nucleophilic addition reaction of aromatic compounds with α-chloroglycidates in the presence of Lewis acid. Cheminform 2002:1781–1790
Liu Y, Liu D, Yang Q, Zhong C, Mi J (2010) Comparative study of separation performance of COFs and MOFs for CH 4/CO2/H2 mixtures. Ind Eng Chem Res 49:2902–2906
Liu L, Tai X, Zhou X (2017) Au(3+)/Au0 supported on chromium(III) terephthalate metal organic framework (MIL-101) as an efficient heterogeneous Catalystfor three-component coupling synthesis of propargylamines. Materials 10:99
Lv H, Zhao H, Cao T, Lin Q, Wang LY, Zhao G (2015) Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J Mol Catal A Chem 400:81–89
Meyer CD, Joiner CS, Stoddart JF (2007) Template-directed synthesis employing reversible imine bond formation. Chem Soc Rev 36:1705–1723
Moro ME, Novillo-Fertrell J, Velazquez MM, Rodriguez LJ (2010) Kinetics of the acid hydrolysis of diazepam, bromazepam, and flunitrazepam in aqueous and micellar systems. J Pharm Sci 80:459–468
Mu M, Wang Y, Qin Y, Yan X, Li Y, Chen LG (2017) Two-dimensional imine-linked covalent organic frameworks as a platform for selective oxidation of olefins. ACS Appl Mater Interfaces 9:22856–22863
Murugan E, Vimala G (2013) Synthesis, characterization, and catalytic activity for hybrids of multi-walled carbon nanotube and amphiphilic poly(propyleneimine) dendrimer immobilized with silver and palladium nanoparticle. J Colloid Interface Sci 396:101–111
Peng Y, Wong WK, Hu Z, Cheng Y, Yuan D, Khan SA, Zhao D (2016) Room temperature batch and continuous flow synthesis of water- stable covalent organic frameworks (COFs). Chem Mater 28:5095–5101
Schestakow M, Muench F, Reimuth C, Ratke L, Ensinger W (2016) Electroless synthesis of cellulose-metal aerogel composites. Appl Phys Lett 108:8896
Shah MT, Balouch A, Sirajuddin, Pathan AA, Umar AA (2017) SiO2 caped Fe3O4 nanostructures as an active heterogeneous catalyst for 4-nitrophenol reduction. Microsystem Technologies 23:1–14
Shen X, Zhu L, Liu G, Yu H, Tang H (2008) Enhanced photocatalytic degradation and selective removal of nitrophenols by using surface molecular imprinted titania. Environ Sci Technol 42:1687–1692
Wang Z, Zhai S, Zhai B, Xiao Z, An Q (2014) Preparation and catalytic properties of nano-Au catalytic materials based on the reduction of 4-nitrophenol. Progress in Chemistry 26:234–247
Wang Z, Su R, Wang D, Shi J, Wang JX, Pu Y, Chen JF (2017) Sulfurized graphene as efficient metal-free catalysts for reduction of 4-nitrophenol to 4-aminophenol. Ind Eng Chem Res 56:13610–13617
Zhao H, Kai C, Zhang H, Hong Z, Han H (2017) Pd-Au heterostructured nanonecklaces with adjustable interval and size as superior catalyst in degradation of 4-nitrophenol. Crystengcomm 19:5686–5691
Zhaoke Z, Takashi T, Tetsuro M (2015) Plasmon-enhanced formic acid dehydrogenation using anisotropic Pd-Au nanorods studied at the single-particle level. J Am Chem Soc 137:948
Zhuang X, Mai Y, Wu D, Zhang F, Feng X (2015) Two-dimensional soft nanomaterials: a fascinating world of materials. Adv Mater 27:403–427
Funding
This work was supported by the National Natural Science Foundation of China and Qinghai Qaidam Saline Lake Chemical Science Research Joint Fund (No. U1607109).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2379 kb)
Rights and permissions
About this article
Cite this article
Hao, S., Li, S. & Jia, Z. Tunable synthesis of Pd/COF-LZU1 for efficient catalysis in nitrophenol reduction. J Nanopart Res 22, 270 (2020). https://doi.org/10.1007/s11051-020-05002-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-020-05002-6