Abstract
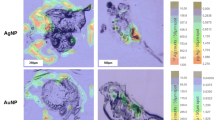
With the wide application of fullerenols in biomedicine, their environmental exposure risks and toxicity to organisms have been extensively studied. However, there is still a lack of knowledge about the distribution of fullerenols in organisms as an important aspect of their mechanism of toxicity. High-resolution matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) is an emerging technology for researching the distribution of molecules in biological tissue samples. Using this high-resolution technique, we map the distribution of fullerenols in zebrafish tissues, and the results suggest that fullerenols enter the gill, intestine, and muscle tissues and even permeate the blood-brain barrier, reaching the brain of zebrafish after aquatic exposure. Moreover, from the MS images of fullerenols, the distribution amount of fullerenols is highest in the gill, followed by that in the intestine and the small amount in muscle and brain tissues. As an emerging environmental pollutant, the establishment of this research method will provide a new method for the study of the environmental toxicity of carbon nanomaterials. Our results also indicated that this high-resolution imaging method could be applied to explore the mechanism of interaction between carbon nanomaterials and biological systems at the cellular level in the future.







Similar content being viewed by others
References
Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials-a short review. Carbohydr Polym. 2010;82(2):227–32. https://doi.org/10.1016/j.carbpol.2010.04.074.
Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol. 2015;11(7):1603–11. https://doi.org/10.1016/j.nano.2015.04.015.
Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012;2012:1–12. https://doi.org/10.1155/2012/751075.
Goncalves DM, Girard D. Evidence that polyhydroxylated C-60 fullerenes (fullerenols) amplify the effect of lipopolysaccharides to induce rapid leukocyte infiltration in vivo. Chem Res Toxicol. 2013;26(12):1884–92. https://doi.org/10.1021/tx4002622.
Grebowski J, Kazmierska P, Krokosz A (2013) Fullerenols as a new therapeutic approach in nanomedicine. Biomed Res Int 1–9. https://doi.org/10.1155/2013/751913
Chaudhuri P, Paraskar A, Soni S, Mashelkar RA, Sengupta S. Fullerenol cytotoxic conjugates for cancer chemotherapy. ACS Nano. 2009;3:2505–14.
Zhao QF, Dong LJ. Ultraviolet protection of fullerenols against Tetrahymena. J Ningbo Univ. 2008;21:474–8.
Zha YY, Yang B, Tang ML, Guo QC, Chen JT, Wen LP, et al. Concentration-dependent effects of fullerenol on cultured hippocampal neuron viability. Int J Nanomedicine. 2012;7:3099–109. https://doi.org/10.2147/IJN.S30934.
Nakagawa Y, Suzuki T, Nakajima K, Inomata A, Ogata A, Nakae D. Effects of N-acetyl-l-cysteine on target sites of hydroxylated fullerene-induced cytotoxicity in isolated rat hepatocytes. Arch Toxicol. 2014;88(1):115–26. https://doi.org/10.1007/s00204-013-1096-3.
Yamawaki H, Iwai N. Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am J Physiol Cell Physiol. 2006;290:1495–502.
Kovac T, Sarkanj B, Klapec T, Borisev I, Kovac M, Nevistic A, et al. Fullerol C60(OH)24 nanoparticles and mycotoxigenic fungi: a preliminary investigation into modulation of mycotoxin production. Environ Sci Pollut Res Int. 2017;24(20):16673–81. https://doi.org/10.1007/s11356-017-9214-z.
Du M, Zhang H, Li J, Yan C, Zhang X, Chang X. Bioaccumulation, depuration, and transfer to offspring of 13C-labeled Fullerenols by Daphnia magna. Environ Sci Technol. 2016;50(19):10421–7. https://doi.org/10.1021/acs.est.6b02596.
Wang CL, Chang X-L, Shi QY, Zhang X. Uptake and transfer of 13C-fullerenols from Scenedesmus obliquus to Daphnia magna in an aquatic environment. Environ Sci Technol. 2018;52(21):12133–41. https://doi.org/10.1021/acs.est.8b03121.
Shi Q, Zhang H, Wang C, Ren H, Yan C, Zhang X, et al. Bioaccumulation, biodistribution and depuration of (13)C-labelled fullerenols in zebrafish through dietary exposure. Ecotoxicol Environ Saf. 2020;191:110173. https://doi.org/10.1016/j.ecoenv.2020.110173.
Shi Q, Wang CL, Zhang H, Chen C, Zhang X, Chang X-L. Trophic transfer and biomagnification of fullerenol nanoparticles in an aquatic food chain. Environ Sci: Nano. 2020. https://doi.org/10.1039/c9en01277j.
Huang XL, Zhang F, Zhu L, Choi KY, Guo N, Guo JX, et al. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano. 2019;7:5684–93.
Georgin D, Czarny B, Botquin M, Mayne-L’Hermite M, Pinault M, Bouchet-Fabre B, et al. Preparation of 14C-labeled multiwalled carbon nanotubes for biodistribution investigations. J Am Chem Soc. 2009;131:14658–9.
Welsher K, Sherlock SP, Dai HJ. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. PANS. 2011;108:8943–8. https://doi.org/10.1073/pnas.1014501108/-/DCSupplemental.
Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. PNAS. 2008;105(5):1410–5. https://doi.org/10.1073/pnas.0707654105.
Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. PNAS. 2008;105(47):18126–31. https://doi.org/10.1073/pnas.0801374105.
McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26(4):606–43. https://doi.org/10.1002/mas.20124.
Harada T, Yuba-Kubo A, Sugiura Y, Zaima N, Hayasaka T, Goto-Inoue N, et al. Visualization of volatile substances in different organelles with an atmospheric-pressure mass microscope. Anal Chem. 2009;81:9153–7.
Carter CL, Jones JW, Farese AM, MacVittie TJ, Kane MA. Inflation-fixation method for lipidomic mapping of lung biopsies by matrix assisted laser desorption/ionization-mass spectrometry imaging. Anal Chem. 2016;88(9):4788–94. https://doi.org/10.1021/acs.analchem.6b00165.
Rodrigues LR, de Oliveira DN, Ferreira MS, Catharino RR. In situ assessment of atorvastatin impurity using MALDI mass spectrometry imaging (MALDI-MSI). Anal Chim Acta. 2014;818:32–8. https://doi.org/10.1016/j.aca.2014.01.050.
Gut Y, Boiret M, Bultel L, Renaud T, Chetouani A, Hafiane A, et al. Application of chemometric algorithms to MALDI mass spectrometry imaging of pharmaceutical tablets. J Pharm Biomed Anal. 2015;105:91–100. https://doi.org/10.1016/j.jpba.2014.11.047.
Zuckschwerdt JB, Nixon CE, Ciner FL, Croley TR. Liquid chromatography/ quadrupole ion trap/time-of-flight determination of the efficacy of drug test kits for rapid screening of food. J Food Prot. 2008;71:1007–14.
Zhang X, Lin Z, Fang J, Liu M, Niu Y, Chen S, et al. An on- line high- performance liquid chromatography- diode- array detector- electrospray ionization- ion- trap- time- of- flight- mass spectrometry- total antioxidant capacity detection system applying two antioxidant methods for activity evaluation of the edible flowers from Prunus mume. J Chromatogr A. 2015;1414:88–102. https://doi.org/10.1016/j.chroma.2015.08.033.
Kurabe N, Igarashi H, Ohnishi I, Tajima S, Inoue Y, Takahashi Y, et al. Visualization of sphingolipids and phospholipids in the fundic gland mucosa of human stomach using imaging mass spectrometry. World J Gastrointest Pathophysiol. 2016;7(2):235–41. https://doi.org/10.4291/wjgp.v7.i2.235.
Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8(5):353–67. https://doi.org/10.1038/nrg2091.
Dai YJ, Jia YF, Chen N, Bian WP, Li QK, Ma YB, et al. Zebrafish as a model system to study toxicology. Environ Toxicol Chem. 2014;33(1):11–7. https://doi.org/10.1002/etc.2406.
Šedo O, Alberti M, Janča J, Havel J. Laser desorption–ionization time of flight mass spectrometry of various carbon materials. Carbon. 2006;44(5):840–7. https://doi.org/10.1016/j.carbon.2005.10.025.
Largy E, Cantais F, Van Vyncht G, Beck A, Delobel A. Orthogonal liquid chromatography- mass spectrometry methods for the comprehensive characterization of therapeutic glycoproteins, from released glycans to intact protein level. J Chromatogr A. 2017;1498:128–46. https://doi.org/10.1016/j.chroma.2017.02.072.
Li F, Wang X, Liu Y, Liu H, Li Z. Dephosphorylation of intact glycoprotein to greatly improve digestion efficiency coupled with matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometric analysis. Anal Chim Acta. 2013;787:140–7. https://doi.org/10.1016/j.aca.2013.05.044.
Wei Y, Zhang Y, Lin Y, Li L, Liu J, Wang Z, et al. A uniform 2,5-dihydroxybenzoic acid layer as a matrix for MALDI-FTICR MS-based lipidomics. Analyst. 2015;140(4):1298–305. https://doi.org/10.1039/c4an01964d.
Stubiger G, Belgacem O. Analysis of lipids using 2,4,6- trihydroxyacetophenone as a matrix for MALDI mass spectrometry. Anal Chem. 2007;79:3206–13.
Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem Biol. 1995;2(6):385–9.
Iwai I. The structure of gut epithelial carp during absorption Tamotsu cells of larval and juvenile of fat and protein. Arch Histol Jap. 1969;30:183–99.
Bai X, Guo Y, Shi Y, Lin J, Tarique I, Wang X, et al. In vivo multivesicular bodies and their exosomes in the absorptive cells of the zebrafish (Danio rerio) gut. Fish Shellfish Immunol. 2019;88:578–86. https://doi.org/10.1016/j.fsi.2019.03.030.
Kashiwada S. Distribution of nanoparticles in the see-through Medaka (Oryzias latipes). Environ Health Perspect. 2006;114:1697–702. https://doi.org/10.1289/ehp.9209.
Mansouri B, Maleki A, Johari SA, Shahmoradi B, Mohammadi E, Shahsavari S, et al. Copper bioaccumulation and depuration in common carp (Cyprinus carpio) following co-exposure to TiO2 and CuO nanoparticles. Arch Environ Contam Toxicol. 2016;71:541–52. https://doi.org/10.1007/s00244-016-0313-5.
Jang MH, Kim WK, Lee SK, Henry TB, Park JW. Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environ Sci Technol. 2014;48:11568–74. https://doi.org/10.1021/es5022813.
Zhu ZJ, Carboni R, Quercio MJ Jr, Yan B, Miranda OR, Anderton DL, et al. Surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish. Small. 2010;6(20):2261–5. https://doi.org/10.1002/smll.201000989.
Yang C, Lee HK, Zhang Y, Jiang LL, Chen ZF, Chung ACK, et al. In situ detection and imaging of PFOS in mouse kidney by matrix-assisted laser desorption/ionization imaging mass spectrometry. Anal Chem. 2019;91(14):8783–8. https://doi.org/10.1021/acs.analchem.9b00711.
Chen QQ, Hu XL, Yin DQ, Wang R. Effect of subcellular distribution on nC60 uptake and transfer efficiency from Scenedesmus obliquus to Daphnia magna. Ecotoxicol Environ Saf. 2016;128:213–21. https://doi.org/10.1016/j.ecoenv.2016.02.026.
Tao XJ, Fortner JD, Zhang B, He YL, Chen YS, Hughes JB. Effects of aqueous stable fullerene nanocrystals (nC60) on Daphnia magna: evaluation of sub-lethal reproductive responses and accumulation. Chemosphere. 2009;77:1482–7. https://doi.org/10.1016/j.chemosphere.2009.10.027.
Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JVK. Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ Toxicol Chem. 2010;29(5):1072–8. https://doi.org/10.1002/etc.124.
Deguchi S, Alargova R, Tsujii GK. Stable dispersions of fullerenes, C60 and C70, in water. Preparation and characterization. Langmuir. 2001;17:6013–7.
Fluri F, Grunstein D, Cam E, Ungethuem U, Hatz F, Schafer J, et al. Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp Neurol. 2015;265:142–51. https://doi.org/10.1016/j.expneurol.2015.01.005.
Kokubo K, Shirakawa S, Kobayashi N, Aoshima H, Oshima T. Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res. 2011;4(2):204–15. https://doi.org/10.1007/s12274-010-0071-z.
Funding
This study was supported by the National Natural Science Foundation of China (41977210).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Chinese Academy of Sciences University and approved by the Animal Ethics Committee of the Institute of Urban Environment, Chinese Academy of Sciences. The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Q., Fang, C., Zhang, Z. et al. Visualization of the tissue distribution of fullerenols in zebrafish (Danio rerio) using imaging mass spectrometry. Anal Bioanal Chem 412, 7649–7658 (2020). https://doi.org/10.1007/s00216-020-02902-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02902-3