Abstract
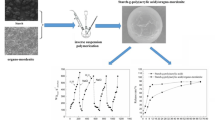
In order to prepare excellent electro-responsive natural hydrogels, in this paper, soluble starch with large amount of hydroxyl groups was selected as raw material. For exploring the enhancement to electro-response of starch hydrogel, starch-Fe(III) composite hydrogels were successfully obtained by a combination of Fe(III) into starch hydrogel in this paper. The composite hydrogels were prepared as A-hydrogels cured in the presence of electric field and B-hydrogels which cured without electric field. The storage modulus of A-hydrogels and B-hydrogels was measured using a dynamic viscoelasticity spectrometer (DMA); the consequent modulus increment (ΔG = GA-GB) and modulus increment sensitivity (ΔG/GB) were analyzed for indicating electro-response of the hydrogels. The physical microstructure of the hydrogels was observed by scanning electron microscopy (SEM). Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA) were used to study the chemical constitution and structure. The results show that there is a coordinate bond between Fe(III) with –OH of starch which leads to a stronger three-dimensional network structure and higher thermal stability of the starch-Fe(III) hydrogel. What’s more, the electro-response of the hydrogels can be affected according to the Fe3+ concentration. When the Fe3+ concentration is 0.15 ~ 0.25 M under the electric field of 0.8 kV mm−1, the starch-Fe(III) hydrogels have the strongest electro-response, the maximum value of modulus increment (ΔG) is 24 kPa, and the modulus increment sensitivity (∆G/GB) is about 90%, far more than pure starch hydrogel.

Starch-iron (III) hydrogel can be obtained with the coordination of –OH and Fe(III) on starch in the presence of electric field or without electric field. The starch-iron (III) hydrogel prepared under the application of an external electric field has a more ordered structure and better mechanical properties.











Similar content being viewed by others
References
Gao C, Ren J, Zhao C, Kong W, Dai Q, Chen Q, Sun R (2016) Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr Polym 151:189–197. https://doi.org/10.1016/j.carbpol.2016.05.075
Andrgie AT, Mekuria SL, Addisu KD, Hailemeskel BZ, Hsu WH, Tsai HC, Lai JY (2019) Non-anticoagulant heparin prodrug loaded biodegradable and injectable thermoresponsive hydrogels for enhanced anti-metastasis therapy. Macromol Biosci 9:1800409. https://doi.org/10.1002/mabi.201800409
Liang Y, Zhao X, Ma P, Guo B, Du Y, Han X (2019) pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci 536:224–234. https://doi.org/10.1016/j.jcis.2018.10.056
Yang C, Liu Z, Chen C, Shi K, Zhang L, Ju XJ, Chu LY (2017) Reduced graphene oxide-containing smart hydrogels with excellent electro-response and mechanical properties for soft actuators. ACS Appl Mater Interfaces 9:15758–15767. https://doi.org/10.1021/acsami.7b01710
Babič M, Vertechy R, Berselli G, Lenarčič J, Castelli VP, Vassura G (2010) An electronic driver for improving the open and closed loop electro-mechanical response of dielectric elastomer actuators. Mechatronics. 20:201–212. https://doi.org/10.1016/j.mechatronics.2009.11.006
Ahn SK, Kasi RM, Kim SC, Sharma N, Zhou Y (2008) Stimuli-responsive polymer gels. Soft Matter 4:1151–1157. https://doi.org/10.1039/b714376a
Mietta JL, Jorge G, Perez OE, Maeder T, Negri RM (2013) Superparamagnetic anisotropic hydrogel connectors exhibiting reversible magneto-piezoresistivity. Sens. Actuators A 192:34–41. https://doi.org/10.1016/j.sna.2012.12.018
Chen WH, Liao WC, Sohn YS, Fadeev M, Cecconello A, Nechushtai R, Willner I (2018) Drug carriers: stimuli-responsive nucleic acid-based polyacrylamide hydrogel-coated metal-organic framework nanoparticles for controlled drug release. Adv Funct Mater 28:1705137. https://doi.org/10.1002/adfm.201870053
Greene AF, Danielson MK, Delawder AO, Liles KP, Li X, Natraj A, Barnes JC (2017) Redox-responsive artificial molecular muscles: reversible radical-based self-assembly for actuating hydrogels. Chem Mater 29:9498–9508. https://doi.org/10.1021/acs.chemmater.7b03635
Elie AG (2010) Electro-conductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials 31:2701–2716. https://doi.org/10.1016/j.biomaterials.2009.12.052
Díaz DD, Kühbeck D, Koopmans RJ (2011) Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem Soc Rev 40:427–448. https://doi.org/10.1039/C005401C
Verber R, Blanazs A, Armes SP (2012) Rheological studies of thermo-responsive diblock copolymer worm gels. Soft Matter 8:9915–9922. https://doi.org/10.1039/c2sm26156a
Panwar V, Lee C, Ko SY, Park JO, Park S (2012) Dynamic mechanical, electrical, and actuation properties of ionic polymer metal composites using PVDF/PVP/PSSA blend membranes. Mater Chem Phys 135:928–937. https://doi.org/10.1016/j.matchemphys.2012.05.081
Yang X, Zhang G, Zhang D (2012) Stimuli responsive gels based on low molecular weight gelators. J Mater Chem 22:38–50. https://doi.org/10.1039/c1jm13205a
Filipcsei G, Fehér J, Zrinyi M (2000) Electric field sensitive neutral polymer gels. J Mol Struct 554:109–117. https://doi.org/10.1016/S0022-2860(00)00564-0
Petcharoen K, Sirivat A (2013) Electrostrictive properties of thermoplastic polyurethane elastomer: effects of urethane type and soft-hard segment composition. Curr Appl Phys 13:1119–1127. https://doi.org/10.1016/j.cap.2013.03.005
Tian L, Deree LT, Botton G, Bhattacharya K (2012) Dielectric elastomer composites. J Mech Phys Solids 60:181–198. https://doi.org/10.1016/j.jmps.2011.08.005
Chen JL, Gao LX, Han XW, Chen T, Luo J, Liu KQ, Gao ZW, Zhang WQ (2016) Preparation and electro-response of chitosan-g-poly (acrylic acid)hydrogel elastomers with interpenetrating network. Mater Chem Phys 169:105–112. https://doi.org/10.1016/j.matchemphys.2015.11.036
Bajpai A, Shukla SK, Bhanu S, Kankane S (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33:1088–1118. https://doi.org/10.1016/j.progpolymsci.2008.07.005
Lin S, Yuan C, Ke A, Quan Z (2008) Electrical response characterization of PVA P(AA/AMPS) IPN hydrogels in aqueous Na2SO4 solution. Sens Actuators B 134:281–286. https://doi.org/10.1016/j.snb.2008.04.045
Kim SJ, Shin SR, Lee SM, Kim IY, Kim SI (2004) Electromechanicl properties of hydrogels based on chitosan and poly(hydroxyethyl methacrylate) in NaCl solution. Smart Mater Struct 13:1036–1039. https://doi.org/10.1088/0964-1726/13/5/008
Kim SJ, Yoon SG, Lee YH, Kim SI (2004) Bending behavior of hydrogels composed of poly (methacrylic acid) and alginate by electrical stimulus. Polym Int 53:1456–1460. https://doi.org/10.1002/pi.1560
An N, Liu H, Ding Y, Zhang M, Tang Y (2011) Preparation and electroactive properties of a PVDF/nano-TiO2 composite film. Appl Surf Sci 257:3831–3835. https://doi.org/10.1016/j.apsusc.2010.12.076
Jun YC, Yoo HJ, Kim YA, Cho JW, Endo M (2010) Electroactive shape memory performance of polyurethane composite having homogeneously dispersed and covalently crosslinked carbon nanotubes. Carbon. 48:1598–1603. https://doi.org/10.1016/j.carbon.2009.12.058
Shi Z, Gao X (2016) Electroconductive natural polymer-based hydrogels. Biomaterials 111:40–54. https://doi.org/10.1016/j.biomaterials.2016.09.020
Garnica-Palafox IM, Sánchez-Arévalo FM (2016) Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr Polym 151:1073–1081. https://doi.org/10.1016/j.carbpol.2016.06.036
Zhao XP, Yin JB (2011) 电场调控的智能软材料 [Smart soft material tuned by electric fields]. Science Press, Beijing
Mahkam M (2010) Modified chitosan cross-linked starch polymers for oral insulin delivery. J Bioact Compat Polym 25:406–418. https://doi.org/10.1177/0883911510369038
Song F, Zhang LM, Shi JF, Li NN (2010) Novel casein hydrogels: formation structure and controlled drug release. Colloids Surf B 79:142–148. https://doi.org/10.1016/j.colsurfb.2010.03.045
Paulino AT, Pereira AGB, Fajardo AR, Erickson K, Kipper MJ, Muniz EIC, Belfiore LA, Tambourgi EB (2012) Natural polymer-based magnetic hydrogels: potential vectors for remote-controlled drug release. Carbohydr Polym 90:1216–1225. https://doi.org/10.1016/j.carbpol.2012.06.051
Chen Y, Zhang JJ, Tan WQ, Wang G, Dong F, Li Q, Guo ZY (2017) Antioxidant activity of inulin derivatives with quaternary ammonium. Starch-Stärke 69:1500294. https://doi.org/10.1002/star.201700046
Pavlovic S, Brandao PRG (2003) Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Miner Eng 16:1117–1122. https://doi.org/10.1016/j.mineng.2003.06.011
Komulainen S, Diaz E, Kärkkäinen J, Pursiainen J, Perämäki P, Lajunen M (2014) Water-soluble oxidized starch in complexation of Fe(III), Cu(II), Ni(II) and Zn(II) ions. React Funct Polym 83:123–131. https://doi.org/10.1016/j.reactfunctpolym.2014.06.007
Gao LX, Chen JL, Han XW, Yan SX, Zhang Y, Zhang WQ, Gao ZW (2015) Electro-response characteristic of starch hydrogel crosslinked with glutaraldehyde. J Biomat Sci-Polym E 26:545–557. https://doi.org/10.1080/09205063.2015.1037587
Lin J, Tang Q, Hu DX, Sun XM, Li QH, Wu JH (2009) Electric field sensitivity of conducting hydrogels with interpenetrating polymer network structure. Colloids Surf A Physicochem Eng Asp 346:177–183. https://doi.org/10.1016/j.colsurfa.2009.06.011
Fan DM, Ma WR, Wang LY, Huang JL, Zhao JX, Zhang H, Chen W (2012) Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Stärke 64:598–606. https://doi.org/10.1002/star.201100200
Bryce DJ, Greenwood CT (1963) The thermal degradation of starch. Part II. The identification by gas chromatography of the minor volatile products produced at 300°C. Starch-Stärke 15:285–290. https://doi.org/10.1002/star.19630150804
Sudharsan K, Chandra Mohan C, Azhagu Saravana Babu P, Archana G, Sabina K, Sivarajan M, Sukumar M (2016) Production and characterization of cellulose reinforced starch (CRT) films. Int J Biol Macromol 83:385–395. https://doi.org/10.1016/j.ijbiomac.2015.11.037
Kandile NG, Nsar AS (2014) New hydrogels based on modified chitosan as metal biosorbent agents. Int J Biol Macromol 64:328–333. https://doi.org/10.1016/j.ijbiomac.2013.12.022
Funding
This work was supported by the Shaanxi Science and Technology Department, Key Research and Development Project under Grant No. 1203010328; projects of the Xi’an Modern Institute of Chemistry under Grant Contract No.204-J-2018-315-4/6, 204-J-2019-0387-3/6-6; and the Fundamental Research Funds for the Central Universities under Grant No. GK201903026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication. The author would like to declare on behalf of her co-authors that the work described was an original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Lin, M., Dai, W. et al. Enhancement of Fe(III) to electro-response of starch hydrogel. Colloid Polym Sci 298, 1533–1541 (2020). https://doi.org/10.1007/s00396-020-04736-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-020-04736-y