Abstract
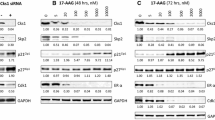
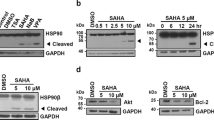
HSP90, one of the molecular chaperones, contributes to protein stability in most living organisms. Previously, we found cleavage of HSP90 by caspase 10 in response to treatment with histone deacetylase inhibitor or proteasome inhibitor in leukemic cell lines. In this study, we investigated this phenomenon in various cell lines and found that HSP90 was cleaved by treatment with SAHA or MG132 in 6 out of 16 solid tumor cell lines. To further investigate the effects of HSP90 cleavage on cells, we introduced mutations to the potential cleavage sites of HSP90β and found that the 294th aspartic acid residue of the protein was mainly cleaved. In the K562 and Mia-PaCa-2 cell lines expressing HSP90β D294A, the cleavage of HSP90 by the treatment with SAHA or MG132 was reduced compared with the K562 and Mia-PaCa-2 cell lines expressing HSP90β WT. Accordingly, cell growth and survival were enhanced by HSP90β D294A expression. Therefore, we suggest that HSP90 cleavage widely occurs in several cell lines, and cleavage of HSP90 may have a potential for one of the mechanisms involved in the anti-tumor effects of known drugs and novel anti-tumor drug candidates.




Similar content being viewed by others
Data availability
The original data are available after contact with the corresponding author.
References
Ali A, Hoeflich KP, Woodgett JR (2001) Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev 101:2527–2540
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280:26729–26734
Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I (2005) Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 23:4152–4161
Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, Raes M, Leveque P, Gallez B, Depuydt M, Collet JF, Calderon PB, Verrax J (2012) Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One 7:e40795
Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, Feron O, Calderon PB (2009) Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol 77:375–383
Beurel E, Grieco SF, Jope RS (2015) Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 148:114–131
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Boltze C, Lehnert H, Schneider-Stock R, Peters B, Hoang Vu C, Roessner A (2004) Withdrawal. HSP90 is a key for telomerase activation and malignant transition in pheochromocytoma. Endocrine 23:229–004–0002-4
Castro JP, Fernando R, Reeg S, Meinl W, Almeida H, Grune T (2019) Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: a novel gain-of-function mechanism. Redox Biol 21:101108
Chen B, Piel WH, Gui L, Bruford E, Monteiro A (2005) The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86:627–637
Chen H, Xia Y, Fang D, Hawke D, Lu Z (2009) Caspase-10-mediated heat shock protein 90 beta cleavage promotes UVB irradiation-induced cell apoptosis. Mol Cell Biol 29:3657–3664
Crawford LJ, Walker B, Irvine AE (2011a) Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5:101–110
Crawford LJ, Walker B, Irvine AE (2011b) Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5:101–110
Curran MP, McKeage K (2009) Bortezomib: a review of its use in patients with multiple myeloma. Drugs 69:859–888
Cusimano A, Azzolina A, Iovanna JL, Bachvarov D, McCubrey JA, D'Alessandro N, Montalto G, Cervello M (2010) Novel combination of celecoxib and proteasome inhibitor MG132 provides synergistic antiproliferative and proapoptotic effects in human liver tumor cells. Cell Cycle 9:1399–1410
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989
Eckschlager T, Plch J, Stiborova M, Hrabeta J (2017) Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci 18. https://doi.org/10.3390/ijms18071414
Ferrarini M, Heltai S, Zocchi MR, Rugarli C (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51:613–619
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Gallegos Ruiz MI, Floor K, Roepman P, Rodriguez JA, Meijer GA, Mooi WJ, Jassem E, Niklinski J, Muley T, van Zandwijk N, Smit EF, Beebe K, Neckers L, Ylstra B, Giaccone G (2008) Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One 3:e0001722
Han YH, Moon HJ, You BR, Park WH (2009) The effect of MG132, a proteasome inhibitor on HeLa cells in relation to cell growth, reactive oxygen species and GSH. Oncol Rep 22:215–221
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR (2000) Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406:86–90
Jackson SE (2013) Hsp90: structure and function. Top Curr Chem 328:155–240
Jayaraj GG, Hipp MS, Hartl FU (2020) Functional modules of the proteostasis network. Cold Spring Harb Perspect Biol 12. https://doi.org/10.1101/cshperspect.a033951
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Kang J, Chen J, Zhang D, Da W, Ou Y (2004) Synergistic killing of human leukemia cells by antioxidants and trichostatin A. Cancer Chemother Pharmacol 54:537–545
Kawai Y, Arinze IJ (2006) Valproic acid-induced gene expression through production of reactive oxygen species. Cancer Res 66:6563–6569
Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Kornberg RD (1999) Eukaryotic transcriptional control. Trends Cell Biol 9:M46–M49
Kumar S, van Raam BJ, Salvesen GS, Cieplak P (2014) Caspase cleavage sites in the human proteome: CaspDB, a database of predicted substrates. PLoS One 9:e110539
Lee P, Murphy B, Miller R, Menon V, Banik NL, Giglio P, Lindhorst SM, Varma AK, Vandergrift WA 3rd, Patel SJ, Das A (2015) Mechanisms and clinical significance of histone deacetylase inhibitors: epigenetic glioblastoma therapy. Anticancer Res 35:615–625
Manoukian AS, Woodgett JR (2002) Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res 84:203–229
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Cocco L, Cervello M, Martelli AM (2014) Multifaceted roles of GSK-3 and Wnt/beta-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 28:15–33
Mills CN, Nowsheen S, Bonner JA, Yang ES (2011) Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front Mol Neurosci 4:47
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76
Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol 143:901–910
Ortiz-Lazareno PC, Bravo-Cuellar A, Lerma-Diaz JM, Jave-Suarez LF, Aguilar-Lemarroy A, Dominguez-Rodriguez JR, Gonzalez-Ramella O, De Celis R, Gomez-Lomeli P, Hernandez-Flores G (2014) Sensitization of U937 leukemia cells to doxorubicin by the MG132 proteasome inhibitor induces an increase in apoptosis by suppressing NF-kappa B and mitochondrial membrane potential loss. Cancer Cell Int 14:13-2867-14-13
Ougolkov AV, Billadeau DD (2006) Targeting GSK-3: a promising approach for cancer therapy? Future Oncol 2:91–100
Park S, Park JA, Yoo H, Park HB, Lee Y (2017) Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS generation and caspase 10-activation in human leukemic cells. Redox Biol 13:470–476
Park S, Park JA, Kim YE, Song S, Kwon HJ, Lee Y (2015) Suberoylanilide hydroxamic acid induces ROS-mediated cleavage of HSP90 in leukemia cells. Cell Stress Chaperones 20:149–157
Park S, Park JA, Jeon JH, Lee Y (2019a) Traditional and novel mechanisms of heat shock protein 90 (HSP90) inhibition in cancer chemotherapy including HSP90 cleavage. Biomol Ther (Seoul) 27:423–434
Park SE, Kim DE, Kim MJ, Lee JS, Rho JK, Jeong SY, Choi EK, Kim CS, Hwang JJ (2019b) Vorinostat enhances gefitinib induced cell death through reactive oxygen species dependent cleavage of HSP90 and its clients in nonsmall cell lung cancer with the EGFR mutation. Oncol Rep 41:525–533
Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM (2007) High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res 67:2932–2937
Powers MV, Workman P (2006) Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer 13(Suppl 1):S125–S135
Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90:65–75
Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A 97:10014–10019
Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW (2001) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of bid and production of reactive oxygen species. Proc Natl Acad Sci U S A 98:10833–10838
Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M (2017) Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis 16:208-017-0593-4
Sato S, Fujita N, Tsuruo T (2000) Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 97:10832–10837
Schopf FH, Biebl MM, Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18:345–360
Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L (2007) An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell 25:151–159
Shirley RB, Kaddour-Djebbar I, Patel DM, Lakshmikanthan V, Lewis RW, Kumar MV (2005) Combination of proteasomal inhibitors lactacystin and MG132 induced synergistic apoptosis in prostate cancer cells. Neoplasia 7:1104–1111
Sreedhar AS, Kalmar E, Csermely P, Shen YF (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15
Wandinger SK, Richter K, Buchner J (2008) The Hsp90 chaperone machinery. J Biol Chem 283:18473–18477
Wayne N, Bolon DN (2007) Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers J Biol Chem 282:35386–35395
Welch WJ (1993) How cells respond to stress. Sci Am 268:56–64
Whitesell L, Santagata S, Lin NU (2012) Inhibiting HSP90 to treat cancer: a strategy in evolution. Curr Mol Med 12:1108–1124
Woodgett JR (1994) Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin Cancer Biol 5:269–275
Acknowledgments
We would like to thank Professor Kyu Lim at Chungnam National University for kindly providing the pancreatic cancer cell line PANC02.
Code availability
Not applicable.
Funding
This research was supported by a grant (2018R1A2B6002504) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and by the Basic Science Research Program (2019R1A6A3A01096936) through the NRF funded by the Ministry of Education in the Republic of Korea.
Author information
Authors and Affiliations
Contributions
Sangkyu Park and Younghee Lee conceived the study and wrote the manuscript. Sangkyu Park, Jae-Hyung Jeon, Jeong-A Park, and Jun-Kyu Choi performed the experiments. Sangkyu Park, Jae-Hyung Jeon, and Younghee Lee analyzed the data and prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors reviewed the manuscript and agreed to publish the data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 251 kb)
Rights and permissions
About this article
Cite this article
Park, S., Jeon, JH., Park, JA. et al. Cleavage of HSP90β induced by histone deacetylase inhibitor and proteasome inhibitor modulates cell growth and apoptosis. Cell Stress and Chaperones 26, 129–139 (2021). https://doi.org/10.1007/s12192-020-01161-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-020-01161-6