Abstract
Thermoresponsive liquid crystal elastomers (LCEs) have a high potential to be used for actuation applications. There has been a substantial amount of literature on synthesis of different LCE networks and their corresponding performance. However, much of the prior work focuses on the experimental aspect of the effects of mesogenic species, crosslinkers, and spacers on the thermal and mechanical response of LCE. Here we have built on these prior studies, and expanded understanding of LCE work capacity and thermal properties to the molecular and network structures by comparing the experimental results to the theoretically predicted values based on a random walk model derived from classical rubber elasticity. A previously developed two stage thiol-acrylate LCE chemistry was used as the model system. On the basis of increasing the chain entropy, we varied crosslinker concentration, crosslinker functionality, and liquid crystal mesogen length and showed that average molecular weight between crosslinks and molecular weight of the Kuhn segment play important roles in controlling the work capacity. The rubber elastic model predicted network performance agreed reasonably well with the experimental results.
Export citation and abstract BibTeX RIS
1. Introduction
As programmable phase-changing materials, thermoresponsive liquid crystal elastomers (LCEs) have great potential to replace bulky, tethered actuators for soft robotic applications. These LCEs consist of both a rigid liquid crystal phase and a soft elastomeric network that can be aligned by mechanical deformation [1], electric field [2], magnetic field [3], or surface patterning [4, 5] to achieve controlled network orientation. Once aligned, a final network crosslinking reaction is commonly introduced to lock in the programmed molecular orientation in an oblate conformation. Upon the input of thermal energy, a phase change occurs from the ordered, liquid crystalline state to an unordered, isotropic state. This phase change results in a macroscopic shape change where the aligned chains contract along the orientation direction and expand perpendicular to the orientation direction due to the Poisson's effect. This mechanical actuation is reversible upon cooling due to the preserved crosslinks in the network.
High work capacity LCEs are desired for efficient actuation. However, progress in this area has historically lagged due to limited monomer availability and few documented methods of synthesizing LCE networks. Recently, there have been significant advances in LCE chemistries, alignment methods, and processing techniques which have improved actuation performance [6–10]. Since these improvements, LCEs have been reported with specific work values ranging from 0.7 to 50 J kg−1 [5, 11–16], and work capacities between 96.9 and 296 kJ m−3 [14, 17–20]. Reported actuation stresses are commonly in the range of 40–400 kPa. Recently, much higher work capacities have been realized through combining liquid crystalline order with additional network structure. By crystallizing the polymer chains, Kim et al reports a work capacity of 730.5 kJ m−3 and a maximum actuation stress of 840 kPa [21]. By introducing an interpenetrating network, the highest LCE work capacity to date has been reported at 1267.7 kJ m−3 with a maximum actuation stress of 2530 kPa [22]. The determination of variables which affect work capacity is difficult due to the wide variety of network chemistries and the disparities between reported data. Among these chemistries, the two-stage thiol-acrylate synthesis has been widely explored due to the facile nature of the chemistry, scalability, and the wide variety of available monomers [10]. Using this chemistry, Yakacki's group looked at the effects of mesogen types, crosslinker content, chain extender length, and crosslinker functionality on the properties of LCE [19, 20, 23].
However, little work has been done on correlating experimental parameters to the theoretical work capacity. Though there has been much theoretical work done on the behavior of LCEs, the complex nature of nematic elastomer theory may be prohibitive to nascent researchers entering the field. There are a myriad of differences between classical rubber elasticity and nematic rubber elasticity, and readers interested in the details of this are directed to the numerous literature examples [24–27]. The most important differences in these theories are due to uniquely liquid crystalline effects, such as molecular anisotropy, soft elasticity, and liquid crystalline phase transitions. Though these liquid crystalline properties impart responsivity into the elastomer, enabling work to be done, it may not be necessary to consider these unique effects when evaluating the impact of network structure on the work capacity.
It is thus the goal of this paper to correlate the LCE network structure with its mechanical and thermal performances both experimentally and theoretically via classical rubber elasticity. Our central hypothesis is that high LCE work capacity can be achieved by enhancing LCE network entropy. To address the entropy change, three variables were manipulated: mesogen type, crosslinker functionality, and crosslinker content. These variables were chosen not only for their ease of experimental permutation, but for their direct effects on theoretical values of Kuhn length, network crosslinking density, and mean end-to-end chain distance. We then compared these theoretical values to the experimentally derived values. This work is distinguished from prior theoretical work in that the contribution of liquid crystalline phase is being intentionally left out; the networks are being examined as purely elastomeric.
2. Materials and methods
2.1. Materials
Two liquid crystal mesogens, 1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene (RM257) and 1,4-Bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene (RM82), were purchased from Wilshire Technologies Inc. (Princeton, NJ, USA). 3,6-Dioxa-1,8-octane-dithiol (EDDT), trimethylolpropane tris(3-mercaptopropionate) (TMPMP), pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), 2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (HHMP), dipropylamine (DPA), and solvents toluene and dichloromethane (DCM) were purchased from Millipore Sigma (St Louis, MO, USA). All reagents were used in their as-received condition.
2.2. Preparation of LCEs
Isotropic polydomain nematic elastomers were prepared following a previously developed two-stage reaction which employs thiol-Michael addition followed by an acrylate photopolymerization [10]. An acrylate to thiol ratio of 1.15:1 was maintained for all formulations. Briefly, mesogen RM257 or RM82 was first dissolved in toluene or DCM respectively, and then heated to 80 °C (for RM257/toluene) and 60 °C (for RM82/DCM) to facilitate dissolution. Thiol spacer EDDT (85 mol%) and crosslinkers PETMP or TMPMP (15 mol%) were then added to the solution and thoroughly mixed. After cooling to room temperature, photoinitiator HHMP and a dilute catalyst solution (1:50 DPA in toluene) were added and thoroughly mixed. The mixtures were then poured into molds and reacted at room temperature for 48 h. After solidification, the solvent was evaporated at 80 °C under vacuum for 48 h.
Polydomain samples were polymerized in a Spectrolight UV light box using broadband UVA light for 10 min at 0.1 mJ cm−2 intensity. Aligned samples were cut into 5 mm × 15 mm × 2 mm rectangles. The samples were stretched uniaxially to 75% of their tested failure strain for each formulation, followed by UV exposure, as stated above.
2.3. Mechanical testing
Polydomain samples of each formulation (n = 4) were cut into 2 mm × 20 mm × 0.2 mm rectangles. Uniaxial tensile tests were performed on an Instron 5900 with a 500 N load cell using a crosshead speed of 0.833 mm s−1. After the test, peak stress and elongation at break were recorded. Elastic modulus was calculated from the linear slope of the first 2% strain of a stress–strain curve to probe the mechanical properties before the liquid crystal alignment occurred.
2.4. Thermal analysis
A differential scanning calorimeter (Mettler-Toledo) (DSC) with the FRS5 sensor under nitrogen purge at 50 ml min−1 was used to determine the glass transition temperature (Tg), and nematic to isotropic transition temperature (Tni). Prior to the experiment, the instrument was calibrated for melting temperature and enthalpy using indium and zinc metal standards. Polydomain LCE samples were cut into specimens with mass of approximately 14 mg and heated from −70 °C to 200 °C at a rate of 20 °C min−1, followed by cooling to −70 °C at 50 °C min−1 for two cycles. All test data were analyzed with STARe SW 10.0 software. Tg was defined as the midpoint of the heat flow before and after the glass transition according to ASTM D7426, and the peak temperature of the endothermic transition was used as Tni.
2.5. Single stroke vertical weight lifting
LCE work capacity of aligned samples was determined using single stroke vertical weight lifting tests. The dimensions and weight of each sample were recorded before hanging the sample from a ring stand using a binder clip. A 100 g weight was hung from the bottom of the sample using another binder clip. Using a convection-based heat gun, the samples were heated to a temperature 30 °C above Tni, ensuring full actuation. During the test, the displacement and response time of the vertical motion were recorded using a long exposure camera (Brinno TimeLapse Camera TLC200 f1.2) with a sampling rate of 1 Hz. LCE work capacity was calculated using W = F × d, where W is the work capacity, F is the force applied by the weight, and d is the distance over which the LCE pulls the weight during actuation.
2.6. Molecular dynamics (MD) simulation
Theoretical estimates of monomer length were obtained using MD simulation. Based on the molecular structure of each repeat unit, the OpenBabel toolkit [28] was used to produce a rough three-dimensional conformation in vacuum, leading to partially coiled structures. Interatomic energies and forces were then calculated more accurately using the density functional tight binding (DFTB) method as implemented in DFTB+ [29, 30], using empirical parameters fitted for organic and biological molecules with extension to include sulfur (3ob-3-1) [31]. Uncoiled structures, better corresponding to theoretical Kuhn segments in a solid polymer where the mesogen-to-mesogen interaction is considered, were obtained by calculating the energy as each monomer was quasi-statically extended by constrained displacement of the terminal atoms. For each displacement, the molecular geometry was relaxed and corresponding energy calculated by minimizing atomic force components within 10−4 eV Å−1 with respect to position by the conjugate gradient method. The Kuhn length was then estimated by identifying the critical length above which the molecular repeat unit was fully extended and exhibited a sharp increase in stored elastic energy.
3. Results and discussion
Though reversible actuation strains of up to 400% have been demonstrated in LCEs, the highest of any known material, their actuation stress is relatively low compared to other actuating materials [19, 32, 33]. To enhance the work capacity of LCEs for robotic applications, this balance of actuation stress and actuation strain, defined as work capacity, should be optimized. The goal of this work is to demonstrate the theoretical basis for the effect of elastomeric network structure on the work capacity of LCEs. Here we present both theoretical calculations based on rubber elastic network theory and experimental calculations to determine the important factors for maximizing work capacity. Utilizing a facile, highly tailorable thiol-acrylate chemistry, we isolate mesogen spacer length, crosslinker amount, and crosslinker functionality to explore the impact of these variables on the work capacity as well as thermal properties.
3.1. Theoretical consideration of LCE work capacity
The work capacity of LCE can be expressed using equation (1) based on the first law of thermodynamics:

where W is work capacity, Q is the energy input, U is the internal energy, and S, T and Cp are entropy, temperature and specific heat, respectively.
Based on equation (1), to maximize work output, the entropy change of the system must be increased, and/or the specific heat of LCE decreased. Given the macromolecular nature of LCE, change in Cp is not always significant. As a result, enhancing work capacity by maximizing the entropy change of the LCE upon transition from the ordered to the disordered state is a reasonable approach. Based on classical rubber elasticity [34], the entropy of an elastomer can be obtained using equation (2)
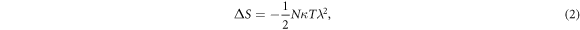
where λ is the stretch ratio of the network, κ is the Boltzmann's constant, T is temperature, and N is the number of chains per volume.
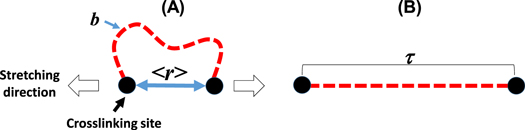
According to equation (2), high entropy change can be realized by increasing the LCE network stretch ratio as well as the number of chains per volume. Assuming affine deformation, the maximum LCE network stretch ratio (λmax) can be calculated by comparing the mean end-to-end chain segment distance  and the average contour length between crosslinks (τ) [35] (equation (3) and figure 1)
and the average contour length between crosslinks (τ) [35] (equation (3) and figure 1)
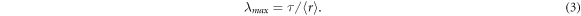
Figure 1. Simplified network structure showing the Kuhn segment (b), contour length between two crosslinks (τ), mean end to end distance  before uniaxial stretching (A) and after uniaxial stretching (B).
before uniaxial stretching (A) and after uniaxial stretching (B).
Download figure:
Standard image High-resolution imageIn equation (3), the fully extended chain contour length between crosslinks (τ) is the summation of the Kuhn's length (b) for all repeat units (n) and equals nb.
The mean end-to-end distance (〈r〉) for random walk model can be obtained using equation (4):
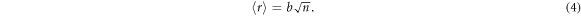
As a result the overall LCE network stretch ratio (λ) relates to the number of repeat units (n) via:
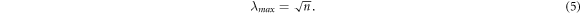
From this analysis, it is clear that the average contour chain length between crosslinks as well as the number of chains that can be activated during stretching greatly influence the entropy change of LCE network and thus the LCE work capacity.
3.2. Experimental LCE synthesis chemistry
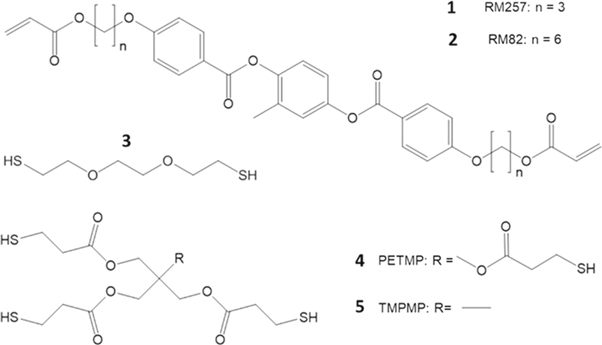
To explore these theoretical relationships, three experimental variables were controlled in the thiol-acrylate LCE network. An excess of diacrylate liquid crystalline mesogen was reacted with a dithiol chain extender and either a trifunctional or tetrafunctional thiol crosslinker, as presented in figure 2. The number of repeat units or the degree of polymerization (n) is varied by changing both the crosslinker content and the crosslinker functionality. The molecular weight of the Kuhn segment was varied by changing the length of the diacrylate mesogen spacer. The two mesogenic monomers had identical rigid core components but contained different alkane chains flanking the cores. With this synthetic strategy, we can isolate the contribution of each experimental variable by constructing comparative networks. The network which will act as the basis for all comparisons consisted 15 mol% excess RM257 reacted with a thiol mixture of 85/15 ratio chain extender to a tetrafunctional crosslinker. Details of the chemistry can be found in the supplemental information table S1 is available online at stacks.iop.org/MFM/3/015002/mmedia.
was varied by changing the length of the diacrylate mesogen spacer. The two mesogenic monomers had identical rigid core components but contained different alkane chains flanking the cores. With this synthetic strategy, we can isolate the contribution of each experimental variable by constructing comparative networks. The network which will act as the basis for all comparisons consisted 15 mol% excess RM257 reacted with a thiol mixture of 85/15 ratio chain extender to a tetrafunctional crosslinker. Details of the chemistry can be found in the supplemental information table S1 is available online at stacks.iop.org/MFM/3/015002/mmedia.
Figure 2. Monomers used in the formulation of LCE networks with varied chain entropy. The liquid crystalline mesogens RM257 (1) or RM82 (2) were reacted with a chain extender EDDT (3) and tetrafunctional crosslinker PETMP (4) or trifunctional crosslinker TMPMP (5) with a molar excess of acrylate.
Download figure:
Standard image High-resolution image3.3. Comparison of LCE network structure and performance based on theoretical and experimental data
To quantitatively compare entropy of different networks, uniaxial tensile tests were performed to extract information about the experimental network in relation to the theoretical network values. First, we determined the theoretical values for  using equation (3). Then we computed the theoretical molecular weight between crosslinks
using equation (3). Then we computed the theoretical molecular weight between crosslinks  using equation (6):
using equation (6):
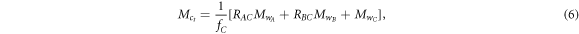
where the subscripts A, B, and C refer to the monomeric components of diacrylate mesogen, chain extender, and crosslinker, respectively. fC is the functionality of the crosslinker, RXY is the molar ratio of two monomeric components, and  is the molecular weight of the monomeric component. This estimation of molecular weight between crosslinks is based on equal stoichiometric ratios between acrylate and thiol functional groups and retains Flory's ideal network assumptions [36]. The number of theoretical repeat units in the contour segment between crosslinks (nt) was estimated by comparing
is the molecular weight of the monomeric component. This estimation of molecular weight between crosslinks is based on equal stoichiometric ratios between acrylate and thiol functional groups and retains Flory's ideal network assumptions [36]. The number of theoretical repeat units in the contour segment between crosslinks (nt) was estimated by comparing  to the molecular weight of each Kuhn's unit
to the molecular weight of each Kuhn's unit  . The results are shown in table 1. Table 1 also shows the Kuhn lengths (b) of both RM257+EDDT and RM82+EDDT which were estimated based on DFTB calculations, which are plotted in figure S1.
. The results are shown in table 1. Table 1 also shows the Kuhn lengths (b) of both RM257+EDDT and RM82+EDDT which were estimated based on DFTB calculations, which are plotted in figure S1.
Table 1. Theoretical network values for LCE networks.
| Mesogen | Crosslinker content (mol%) | fC | 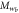 (g mol−1) (g mol−1) |
b(nm) | 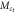 (g mol−1) (g mol−1) |
nt | τt (nm) |
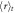 (nm) (nm) |
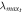 |
|---|---|---|---|---|---|---|---|---|---|
| RM257 | 4.7 | 4 | 771 | 4.4 | 1873 | 2.4 | 10.7 | 6.9 | 1.6 |
| RM257 | 7.0 | 4 | 771 | 4.4 | 1312 | 1.7 | 7.5 | 5.7 | 1.3 |
| RM257 | 9.3 | 4 | 771 | 4.4 | 1034 | 1.3 | 5.9 | 5.1 | 1.2 |
| RM257 | 23.3 | 4 | 771 | 4.4 | 565 | 0.7 | 3.2 | 3.8 | 0.9 |
| RM257 | 7.0 | 3 | 771 | 4.4 | 1719 | 2.2 | 9.8 | 6.6 | 1.5 |
| RM82 | 7.0 | 4 | 855 | 5.4 | 1473 | 1.7 | 9.3 | 7.1 | 1.3 |
| RM82 | 7.0 | 3 | 855 | 5.4 | 1934 | 2.3 | 12.2 | 8.1 | 1.5 |
The experimental maximum stretch ratio and average molecular weight between crosslinks
and average molecular weight between crosslinks  values were obtained by performing uniaxial tensile tests.
values were obtained by performing uniaxial tensile tests.  values were obtained using the classical rubber theory [34, 37]:
values were obtained using the classical rubber theory [34, 37]:
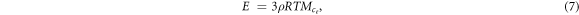
where E is the elastic modulus of the LCE, T is temperature, R is the gas constant, and ρ is the specific density. The molecular weight and Kuhn length were assumed the same as the theoretical values. The experimental degree of crosslinking (ne) was calculated using the same method as described in the theoretical section above for table 1.
Comparing the theoretical and experimental values here gives great insight to the generalization of an LCE as an ideal rubber. Within both the theoretical and the experimental values, the expected trends hold. As the crosslinker content was increased, both Mc and λmax decreased. As the crosslinker functionality was increased, both Mc and λmax decreased. As the mesogen spacer length was increased, both Mc and λmax increased. These qualitative relationships hold true to equation (1) and our central hypothesis that increasing chain length increases the system entropy.
Figure 3 shows the comparison between experimental and theoretical values for Mc and λmax. The results indicate that the average molecular weight between crosslinks shows good agreement between theory predicted and experimentally measured values (figure 3(A)). However, the theoretical ultimate extension ratios significantly underestimate the experimental values due to the deviation of these LCE systems from the ideal rubber assumption.
Figure 3. (A) Comparison between the theoretical and experimental molecular weight between crosslinks, and (B) comparison between theoretical and experimental maximum extension.
Download figure:
Standard image High-resolution imageIn addition to the mechanical performance, table 2 also shows the effects of network properties on the two thermal transitions in LCE: glass transition and nematic to isotropic transition. The glass transition is a second order transition associated with the relaxation of the chains in the amorphous phase, and the magnitude of the glass transition temperature (Tg) reflects the level of constraints that the chains are experiencing. Higher LCE network crosslinking density restricts the segmental relaxation in LCEs leading to higher Tg. The experimental results from changing the crosslinker content confirm the theoretical predictions that higher crosslinker content or higher crosslinking density enhanced the magnitude of Tg. This finding is in agreement with previous experimental results [20]. Additionally, higher crosslinker functionality increased the magnitude of Tg. Based on chain length, the RM82 network with tri-functional crosslinker should yield the lowest Tg and thus highest entropy. Experimentally, we find that this network is evenly matched with the RM257 network with the tri-functional crosslinker, illustrating that mesogen spacer length has a small effect on at least the thermal transitions of the network, and possibly the chain entropy as well. This conclusion is consistent with previously reported results, which indicate that increasing chain extender length does not affect the Tg [19]. Lastly, the nematic to isotropic transition temperature (Tni) also decreased with increasing crosslinking density. This is likely due to the domain size decrease induced by high crosslinking density, which in turn reduced the extended crystal thickness in the nematic phase. The thinner nematic phase crystal thickness in turn leads to a lower Tni according to the Gibbs–Thomson effect [38].
Table 2. Experimental data for LCE network structures.
| Mesogen | Crosslinker content (mol%) | fC | 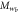 (g mol−1) (g mol−1) |
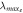
|
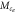 (g mol−1) (g mol−1) |
ne | Tg (°C) | Tni (°C) |
|---|---|---|---|---|---|---|---|---|
| RM257 | 4.7 | 4 | 771 | 3.2 | 1412 | 1.8 | 3.7 | 70.1 |
| RM257 | 7.0 | 4 | 771 | 2.6 | 913 | 1.2 | 5.8 | 71.8 |
| RM257 | 9.3 | 4 | 771 | 1.9 | 784 | 1.0 | 7.5 | 68.4 |
| RM257 | 23.3 | 4 | 771 | 1.9 | 135 | 0.2 | 18.2 | 66.0 |
| RM257 | 7.0 | 3 | 771 | 3.1 | 1782 | 2.3 | 0.7 | 65.9 |
| RM82 | 7.0 | 4 | 855 | 3.9 | 1177 | 1.4 | −9.2 | 91.7 |
| RM82 | 7.0 | 3 | 855 | 4.4 | 1420 | 1.7 | −10.3 | 86.0 |
3.4. Effect of variables on work capacity
Here, we return to the central hypothesis of the paper: the network work capacity can be increased by increasing the chain entropy. While qualitative and quantitative manipulation of chain entropy was demonstrated in the preceding sections, the effect on work capacity has not yet been proven. Based on equation (1), changing the entropy should change the amount of work done by a phase change. As discussed in section 3.1, work capacity should vary with entropy as a function of two main variables: the ultimate extension (λmax) and the molecular weight between crosslinks (Mc). Work capacity of each LCE network is determined by a weight lifting test detailed in the methods. As the weight attached increases, the work capacity increases as well. Figure 4 compares the work capacity of different LCE networks as a function of both theoretical and experimental ultimate extension, or molecular weight between crosslinks. Work capacity is measured for two loading stresses: σ = 1 MPa and σ = 2 MPa. From equations (1)–(3), (5), and assuming a constant Cp, we can expect the following relationship:
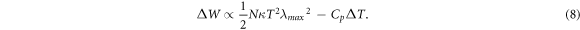
Figure 4. Comparison of the theoretical (A) and experimental (B) values of ultimate extension on the work capacity of LCE networks. The effects of molecular weight between crosslinks (Mc) based on both theoretically (C) and experimentally (D) determined results are also shown. The work capacity is shown for loading stresses of 1 MPa (red circles) or 2 MPa (blue circles).
Download figure:
Standard image High-resolution imageTherefore, λmax should have a power law relationship with work capacity. Indeed figures 4(A), (B) show a power relationship for the theoretical extension ratio. Figures 4(C), (D) show that the increase in Mc generally increased the work capacity of LCE even though the theoretical data seemed to show maxima at Mc around 1500 g mol−1. One possible explanation is the quality of mesogen alignment, which is not accounted for in classical rubber elasticity, as it is uniquely liquid crystalline. It is well known that the quality of alignment affects the amount of contraction produced by the LCE [39, 40]. Therefore the quality of the liquid crystalline alignment is a large enough factor to influence the patterns of small sample sets. Regardless of this liquid crystalline contribution, classical rubber elasticity did predict the correct general trends for the LCE work capacity.
Therefore, using classical rubber elasticity is a valid method to guide the design of high work capacity LCEs, and can perhaps be applied to other traits, such as power. However, the effects of liquid crystalline behavior cannot be neglected if specific values are targeted for work capacity, as the liquid crystalline contributions to the entropy are too high to discount. It should be noted that the theoretical maximum work capacity demonstrated in figures 4(a) and (c) of approximately 150 kJ m−3 should not be taken as a benchmark maximum work capacity value. Though the maximum for the variables tested in this work, there are many factors which contribute to the maximization of work capacity not covered in this work. These factors include applied stress, chain orientation, energy conversion efficiency, and breaking strength, among others [17, 41–44]. However, the central finding of this paper should remain consistent over all networks, that maximizing the entropy though network structure can maximize the work capacity of a given system.
4. Conclusions
In this paper, we investigated the fidelity of LCE network parameters and work capacity to classical rubber elasticity models. The chain entropy of a given LCE network can be used as a relative benchmark to compare formulations and design maximum work capacity networks. We showed that molecular weight between crosslinks and ultimate extension are good indicators of work capacity both theoretically and experimentally. Importantly, the central findings of this work should be applicable to all types of LCEs, regardless of the network chemistry or the type of liquid crystalline mesogen attachment. However, using classical rubber elasticity alone cannot accurately predict precise values for network parameters, especially dynamic variables such as ultimate extension. In these cases, the liquid crystal contribution becomes too high to gauge appropriate values, though the trends remain intact. It is the finding of this work that classical rubber elasticity is a good gauge of relative work capacity of LCE networks and can be used to inform the design of LCE networks.
Acknowledgments
Financial support of this work was provided by the Independent Research and Development (IRaD) fund provided by the Research and Exploratory Development Mission Area of the Johns Hopkins University/Applied Physics Laboratory. We thank Professor Christopher K Yakacki from University of Colorado Denver for the support of this work.