Abstract
The nuclear receptor-binding SET domain (NSD) family of histone methyltransferases is associated with various malignancies, including aggressive acute leukemia with NUP98-NSD1 translocation. While NSD proteins represent attractive drug targets, their catalytic SET domains exist in autoinhibited conformation, presenting notable challenges for inhibitor development. Here, we employed a fragment-based screening strategy followed by chemical optimization, which resulted in the development of the first-in-class irreversible small-molecule inhibitors of the nuclear receptor-binding SET domain protein 1 (NSD1) SET domain. The crystal structure of NSD1 in complex with covalently bound ligand reveals a conformational change in the autoinhibitory loop of the SET domain and formation of a channel-like pocket suitable for targeting with small molecules. Our covalent lead—compound BT5—demonstrates on-target activity in NUP98-NSD1 leukemia cells, including inhibition of histone H3 lysine 36 dimethylation and downregulation of target genes, and impaired colony formation in an NUP98-NSD1 patient sample. This study will facilitate the development of the next generation of potent and selective inhibitors of the NSD histone methyltransferases.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The crystal structure of NSD1 (PDB code no. 3OOI) was used as the search model to determine the structures reported in this study. The crystal structures of NSD1 and NSD1 in complex with BT3 have been deposited in the PDB under the accession codes 6KQP and 6KQQ, respectively. Source data are provided with this paper.
References
Bennett, R. L., Swaroop, A., Troche, C. & Licht, J. D. The role of nuclear receptor-binding SET domain family histone lysine methyltransferases in cancer. Cold Spring Harb. Perspect. Med. 7, a026708 (2017).
Wagner, E. J. & Carpenter, P. B. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell Biol. 13, 115–126 (2012).
Morishita, M. & di Luccio, E. Cancers and the NSD family of histone lysine methyltransferases. Biochim. Biophys. Acta 1816, 158–163 (2011).
Job, B. et al. Genomic aberrations in lung adenocarcinoma in never smokers. PLoS ONE 5, e15145 (2010).
Bianco-Miotto, T. et al. Global levels of specific histone modifications and an epigenetic gene signature predict prostate cancer progression and development. Cancer Epidemiol. Biomarkers Prev. 19, 2611–2622 (2010).
Lawrence, M. S. et al. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582 (2015).
Hollink, I. H. I. M. et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood 118, 3645–3656 (2011).
Shiba, N. et al. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosomes Cancer 52, 683–693 (2013).
Deshpande, A. J. et al. AF10 regulates progressive H3K79 methylation and HOX gene expression in diverse AML subtypes. Cancer Cell 26, 896–908 (2014).
Wang, G. G., Cai, L., Pasillas, M. P. & Kamps, M. P. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat. Cell Biol. 9, 804–812 (2007).
Qiao, Q. et al. The structure of NSD1 reveals an autoregulatory mechanism underlying histone H3K36 methylation. J. Biol. Chem. 286, 8361–8368 (2011).
Shen, Y. et al. Identification of LEM-14 inhibitor of the oncoprotein NSD2. Biochem. Biophys. Res. Commun. 508, 102–108 (2019).
Coussens, N. P. et al. High-throughput screening with nucleosome substrate identifies small-molecule inhibitors of the human histone lysine methyltransferase NSD2. J. Biol. Chem. 293, 13750–13765 (2018).
Tisi, D. et al. Structure of the epigenetic oncogene MMSET and inhibition by N-alkyl sinefungin derivatives. ACS Chem. Biol. 11, 3093–3105 (2016).
Morrison, M. J. et al. Identification of a peptide inhibitor for the histone methyltransferase WHSC1. PLoS ONE 13, e0197082 (2018).
Hopkins, A. L., Keserü, G. M., Leeson, P. D., Rees, D. C. & Reynolds, C. H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 13, 105–121 (2014).
Degani, Y., Neumann, H. & Patchornik, A. Selective cyanylation of sulfhydryl groups. J. Am. Chem. Soc. 92, 6969–6971 (1970).
Fick, R. J. et al. Sulfur-oxygen chalcogen bonding mediates AdoMet recognition in the lysine methyltransferase SET7/9. ACS Chem. Biol. 11, 748–754 (2016).
Abdeldayem, A., Raouf, Y. S., Constantinescu, S. N., Moriggl, R. & Gunning, P. T. Advances in covalent kinase inhibitors. Chem. Soc. Rev. 49, 2617–2687 (2020).
McGregor, L. M., Jenkins, M. L., Kerwin, C., Burke, J. E. & Shokat, K. M. Expanding the scope of electrophiles capable of targeting K-Ras oncogenes. Biochemistry 56, 3178–3183 (2017).
Strelow, J. M. A perspective on the kinetics of covalent and irreversible inhibition. SLAS Discov. 22, 3–20 (2017).
Rogawski, D. S. et al. Two loops undergoing concerted dynamics regulate the activity of the ASH1L histone methyltransferase. Biochemistry 54, 5401–5413 (2015).
Martinez Molina, D. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Dart, M. L. et al. Homogeneous assay for target engagement utilizing bioluminescent thermal shift. ACS Med. Chem. Lett. 9, 546–551 (2018).
Borkin, D. et al. Pharmacologic inhibition of the menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27, 589–602 (2015).
Xu, H. et al. NUP98 fusion proteins interact with the NSL and MLL1 complexes to drive leukemogenesis. Cancer Cell 30, 863–878 (2016).
Shima, H. et al. Ring1A and Ring1B inhibit expression of Glis2 to maintain murine MOZ-TIF2 AML stem cells. Blood 131, 1833–1845 (2018).
Edmunds, J. W., Mahadevan, L. C. & Clayton, A. L. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 27, 406–420 (2008).
Hansen, R. et al. The reactivity-driven biochemical mechanism of covalent KRASG12C inhibitors. Nat. Struct. Mol. Biol. 25, 454–462 (2018).
Eram, M. S. et al. Kinetic characterization of human histone H3 lysine 36 methyltransferases, ASH1L and SETD2. Biochim. Biophys. Acta 1850, 1842–1848 (2015).
Wu, H. et al. Structural biology of human H3K9 methyltransferases. PLoS ONE 5, e8570 (2010).
Eram, M. S. et al. Trimethylation of histone H3 lysine 36 by human methyltransferase PRDM9 protein. J. Biol. Chem. 289, 12177–12188 (2014).
Del Rizzo, P. A. et al. SET7/9 catalytic mutants reveal the role of active site water molecules in lysine multiple methylation. J. Biol. Chem. 285, 31849–31858 (2010).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Jo, S. Y., Granowicz, E. M., Maillard, I., Thomas, D. & Hess, J. L. Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood 117, 4759–4768 (2011).
Acknowledgements
This work was funded by National Institute of Health (NIH) grant nos. R01 CA226759 and CA207272 to T.C., and grant nos. 1R01 CA160467 and R01 CA244254 to J.G.; Leukemia & Lymphoma Society (LLS) Translational Research Program grant nos. 6111-14 and 6564-19 to T.C., 6485-16 and 6579-20 to J.G. and LLS Scholar grant nos. 1340-17 to T.C. and 1215-14 to J.G. C.A.H. is supported by Chemistry Biology Interface Training Program (no. T32GM008597). J.G. is Rogel Scholar at the University of Michigan. This work was also supported by the Chemotherapy Foundation (A.F.) and NIH grant nos. R35 CA210065 (to A.F.) and P30 CA013696 (Flow Cytometry Shared Resource). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant no. 085P1000817). The NUP98-NSD1 construct was a kind gift from A. J. Deshpande. The MOZ-TIF2 construct was provided by I. Kitabayashi and the NUP98-HOXA9 cells were provided by H. Xu.
Author information
Authors and Affiliations
Contributions
T.C. and J.G. are responsible for initiating and supervising the entire project. H.H., S.Z. and M.A.P. synthesized the compounds. H.J.C. carried out the structural biology studies. C.A.H., H.L., J.N., D.S.R., C.N., J.A. and A.H. performed the biochemical studies. S.S., K.K., P.G.A., H.M. and T.P. performed the cell biology studies. A.M., M.L.S. and A.F. provided the reagents and advised the study. All authors contributed to data analysis and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.H., C.A.H., S.Z., H.J.C., M.A.P., J.G. and T.C. are coinventors on a patent application for NSD1 inhibitors. T.C. and J.G. received prior support from Kura Oncology for an unrelated project and received licensing royalties from Kura Oncology. A.F. is consulting for Ayala Pharmaceuticals and SpringWorks Therapeutics; he received previous research support from Pfizer, Bristol Myers Squibb, Merck and Eli Lilly, as well as patent and reagent licensing royalties from Novartis, EMD Millipore and Applied Biological Materials.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
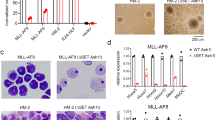
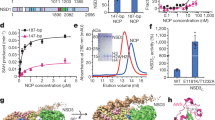
Extended Data Fig. 1 Crystal structure of NSD1 SET domain and mapping of the binding of BT2 using NMR.
a, Superposition of the crystal structure of NSD1 SET domain (residues 1863–2085) determined in this work (magenta) onto the previously described crystal structure of NSD1 SET (PDB code 3OOI, green). Positions of the N- and C- termini are labeled and SAM is in blue sticks. b, Ribbon (left) and surface (right) representations of NSD1 SET domain (residues 1863–2085) with mapped residues undergoing strong chemical shift perturbations (ΔσHN > 0.05ppm or ΔσN > 0.5ppm) upon binding of BT2 (shown in red).
Extended Data Fig. 2 Crystal structure of NSD1 SET domain in complex with covalently bound compound BT3.
a, The crystal structure of NSD1 SET domain (residues 1863–2085) with bound BT3 (blue), SAM (pale blue) and mapped residues undergoing strong chemical shift perturbations (ΔσHN > 0.05ppm or ΔσN > 0.5ppm) upon binding of BT3 (shown in red). Disulfide-bond dimer of BT3 found in the structure (pale green). The dimer binds in a site that is distant from inhibitor binding and lack of chemical shift perturbations in this area indicates that this represents a crystallization artifact. b, Same as in panel a rotated by 90 deg.
Extended Data Fig. 3 BT3 and BT5 bind to the same site on NSD1 SET domain.
a, b, Comparison of the two different fragments of 1H-15N HSQC spectra of 150 μM NSD1 SET domain (black), with 500 μM compound BT3 (blue) or 300 μM compound BT5 (red). Selected residues perturbed by both compounds BT3 and BT5 are boxed. c, The crystal structure of NSD1-BT3 complex showing chemical shift perturbations (ΔσHN > 0.05ppm or ΔσN > 0.5ppm) upon binding of BT3 (shown in red). Position of two cysteine residues (C2070 and C2072) involved in Zn coordination and strongly perturbed upon binding of BT3 is shown.
Extended Data Fig. 4 Compound BT5 does not bind covalently to NSD1 C2062A SET domain.
a, MS spectra of 1 μM wild-type NSD1 SET domain incubated with DMSO (left) or 16 μM BT5 (right) for 2 h showing 51% covalent engagement with BT5. b, MS spectra of 1 μM NSD1 C2062A SET domain incubated with DMSO (left) or 16 μM BT5 (right) for 2 h. No covalent engagement of BT5 is observed (expected mass 30,066 Da). Representative spectra shown are from two independent experiments (n = 2).
Extended Data Fig. 5 Treatment with BT5 lacks engagement with NSD2 and NSD3 in cells.
CETSA assay in HEK293T cells transfected with FLAG-NSD2 SET (top) or FLAG-NSD3 SET (bottom) constructs treated with DMSO or 5 μM BT5. Cell lysates were incubated for 3 min at indicated temperatures. Experiments were performed two times.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Table 1 and Notes
Source data
Source Data Fig. 5
Uncropped gels for panels a, d, e, f
Source Data Extended Data Fig. 5
Uncropped gels
Rights and permissions
About this article
Cite this article
Huang, H., Howard, C.A., Zari, S. et al. Covalent inhibition of NSD1 histone methyltransferase. Nat Chem Biol 16, 1403–1410 (2020). https://doi.org/10.1038/s41589-020-0626-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0626-6
This article is cited by
-
NSD1 promotes esophageal cancer tumorigenesis via HIF1α signaling
Cell Biology and Toxicology (2023)
-
Target validation and structure-based virtual screening to Discover potential lead molecules against the oncogenic NSD1 histone methyltransferase
In Silico Pharmacology (2023)
-
NSD1 gene evolves under episodic selection within primates and mutations of specific exons in humans cause Sotos syndrome
BMC Genomics (2022)
-
Small molecule targeting of chromatin writers in cancer
Nature Chemical Biology (2022)
-
The role of NSD1, NSD2, and NSD3 histone methyltransferases in solid tumors
Cellular and Molecular Life Sciences (2022)