Abstract—
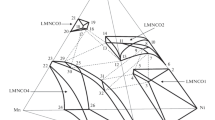
This paper presents a topological analysis of phase equilibria in the Li2O–Al2O3–Ni–Co–O system. Using the fragmentary experimental data available, we construct isothermal phase diagrams of the Li2O–Al2O3–Nc–O (Nc = Ni + Co) and Li2O–Al2O3–NiO1 + x–CoO1 + x (0 ≤ x < 1) systems. Data are presented on phase equilibria involving Li(Ni,Co,Al)O2 (α-NaFeO2 structure), (Li,Ni,Co,Al)Al2O4 (spinel structure), (Li,Ni,Co)O (rock-salt structure), and (Li,Ni,Co)3O4 (spinel structure) solid solutions, whose homogeneity regions are evaluated.


Similar content being viewed by others
REFERENCES
Li, W., Liu, X., Celio, H., Smith, P., Dolocan, A., Chi, M., and Manthiram, A., Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability, Adv. Energ. Mater., 2018, vol. 8, no. 15, paper 703154. https://doi.org/10.1002/aenm.201703154
Wang, Q., Zhang, L., Zhao, P., and Du, Z., Facile synthesis of a high-capacity LiNi0.8Co0.15Al0.05O2 nanoplate cathode with a {010} orientation for lithium-ion batteries, Int. J. Electrochem. Sci., 2018, vol. 13, pp. 10382–10389.https://doi.org/10.20964/2018.11.15
Purwanto, A., Yudha, C.S., Ubaidillah, U., Widiyandari, H., Ogi, T., and Haerudin, H., NCA cathode material: synthesis methods and performance enhancement efforts, Mater. Res. Express, 2018, vol. 5, paper 122001.https://doi.org/10.1088/2053-1591/aae167
Ding, N., Wang, X., Hou, Y., Wang, S., Li, X., Fam, D.W.H., Zong, Y., and Liu, Z., Rational design of a high-energy LiNi0.8Co0.15Al0.05O2 cathode for Li-ion batteries, Solid State Ionics, 2018, vol. 323, pp. 72–77.https://doi.org/10.1016/j.ssi.2018.05.020
Liu, W., Qin, M., Gao, C., Yu, D., and Yue, Y., Green and low-cost synthesis of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries, Mater. Lett., 2019, vol. 246, pp. 153–156.https://doi.org/10.1016/j.matlet.2019.03.064
Cao, C., Zhang, J., Xie, X., and Xia, B., A novel method for the modification of LiNi0.8Co0.15Al0.05O2 with high cycle stability and low pH, J. Solid State Electrochem., 2019, vol. 23, no. 5, pp. 1351–1358.https://doi.org/10.1007/s10008-019-04216-6
He, X., Han, G., Lou, S., Du, L., Xu, X., Du, C., Cheng, X., Zuo, P., Ma, Y., Huo, H., and Yin, G., Improved electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material by coating of graphene nanodots, J. Electrochem. Soc., 2019, vol. 166, no. 6, pp. A1038–A1044.https://doi.org/10.1149/2.0541906jes
Liang, M., Sun, Y., Song, D., Shi, X., Han, Y., Zhang, H., and Zhang, L., Superior electrochemical performance of quasi-concentration-gradient LiNi0.8Co0.15Al0.05O2 cathode material synthesized with multi-shell precursor and new aluminum source, Electrochim. Acta, 2019, vol. 300, pp. 426–436.https://doi.org/10.1016/j.electacta.2019.01.125
Jo, M., Noh, M., Oh, P., Kim, Y., and Cho, J., A new high power LiNi0.81Co0.1Al0.09O2 cathode material for lithium-ion batteries, Adv. Energ. Mater., 2014, vol. 4, no. 13, paper 301583.https://doi.org/10.1002/aenm.201301583
Chen, W., Li, Y., Yang, D., Feng, X., Guan, X., and Mi, L., Controlled synthesis of spherical hierarchical LiNi1 –x–yCoxAlyO2 (0 < x, y < 0.2) via a novel cation exchange process as cathode materials for high-performance lithium batteries, Electrochim. Acta, 2016, vol. 190, pp. 932–938.https://doi.org/10.1016/j.electacta.2016.01.024
Zhou, P., Meng, H., Zhang, Z., Chen, C., Lu, Y., Cao, J., Cheng, F., and Chen, J., Stable layered Ni-rich LiNi0.9Co0.07Al0.03O2 microspheres assembled with nanoparticles as high-performance cathode materials for lithium-ion batteries, J. Mater. Chem. A, 2017, vol. 5, no. 6, pp. 2724–2731.https://doi.org/10.1039/C6TA09921A
Cao, H., Xia, B., Xu, N., and Zhang, C., Structural and electrochemical characteristics of Co and Al co-doped lithium nickelate cathode materials for lithium-ion batteries, J. Alloys. Compd., 2004, vol. 376, nos. 1–2, pp. 282–286.https://doi.org/10.1016/j.jallcom.2004.01.008
Ju, S.H., Kim, J.H., and Kang, Y.C., Electrochemical properties of LiNi0.8Co0.2 –xAlxO2 (0 ≤ x ≤ 0.1) cathode particles prepared by spray pyrolysis from the spray solutions with and without organic additives, Met. Mater. Int., 2010, vol. 16, no. 2, pp. 299–303.https://doi.org/10.1007/s12540-010-0421-0P
Zhu, X.-J., Liu, H.-X., Gan, X.-Y., Cao, M.-H., Zhou, J., Chen, W., Xu, Q., and Quyang, S.-X., Preparation and characterization of LiNi0.80Co0.20 –xAlxO2 as cathode materials for lithium ion batteries, J. Electroceram., 2006, vol. 17, nos. 2–4, pp. 645–649.https://doi.org/10.1007/s10832-006-6705-6
Vogler, C., Hemmer, G., Arnold, G., Trépo, A., and Wohlfahrt-Mehrens, M., Lithium nickel oxide Li(Ni0.75Al0.17Co0.08)O2 as cathode material for lithium ion batteries, Ionics, 1999, vol. 5, nos. 5–6, pp. 421–425. https://doi.org/10.1007/BF02376008
Madhavi, S., Subba Rao, G.V., Chowdari, B.V.R., and Li, S.F.Y., Effect of aluminium doping on cathodic behaviour of LiNi0.7Co0.3O2, J. Power Sources, 2001, vol. 93, pp. 156–162.
Kalyani, P., Kalaiselvi, N., Renganathan, N.G., and Raghavan, M., Studies on LiNi0.7Al0.3 –xCoxO2 solid solutions as alternative cathode materials for lithium batteries, Mater. Res. Bull., 2004, vol. 39, no. 1, pp. 41–54.https://doi.org/10.1016/j.materresbull.2003.09.021
Reddy, M.V., Subba Rao, G.V., and Chowdary, B.V.R., Preparation and characterization of LiNi0.5Co0.5O2 and LiNi0.5Co0.4Al0.1O2 by molten salt synthesis for Li ion batteries, J. Phys. Chem. C, 2007, vol. 111, no. 31, pp. 11712–11720.https://doi.org/10.1021/jp0676890
Chang, Z.-R., Yu, X., Tang, H.-W., Wei, W.-Q., and Dai, D.-M., Influence of Al doping content on the structure and electrochemical properties of LiNi1/3Co2/3xAlxO2, Acta Phys.-Chim. Sin., 2010, vol. 26, no. 3, pp. 567–572.https://doi.org/10.3866/PKU.WHXB20100247
Castro-García, S., Castro-Couceiro, A., Señarís–Rodríguez, M.A., Soulette, F., and Julien, C., Influence of aluminum doping on the properties of LiCoO2 and LiNi0.5Co0.5O2 oxides, Solid State Ionics, 2003, vol. 156, nos. 1–2, pp. 15–26.https://doi.org/10.1016/S0167-2738(02)00570-2
Adipranoto, D.S., Ishigaki, T., Hoshikawa, A., Iwase, K., Yonemura, M., Mori, K., Kamiyama, Y., Morii, Y., and Hayashi, M., Neutron diffraction studies on structural effect for Ni-doping in LiCo1 −xNixO2, Solid State Ionics, 2014, vol. 262, pp. 92–97.https://doi.org/10.1016/j.ssi.2013.11.014
Gao, J., Shi, S., Xiao, R., and Li, H., Synthesis and ionic transport mechanisms of α-LiAlO2, Solid State Ionics, 2016, vol, 286, pp. 122–134.https://doi.org/10.1016/j.ssi.2015.12.028
Dahéron, L., Dedryvère, R., Martinez, H., Flahaut, D., Ménétrier, M., Delmas, C., and Gonbeau, D., Possible explanation for the efficiency of Al-based coatings on LiCoO2: surface properties of LiCo1 – xAlxO2 solid solution, Chem. Mater., 2009, vol. 21, no. 23, pp. 5607–5616.https://doi.org/10.1021/cm901972e
Shinova, E., Zhecheva, E., and Stoyanova, R., Formation of LiAlyNi1 –yO2 solid solutions under high and atmospheric pressure, J. Solid State Chem., 2006, vol. 179, no. 10, pp. 3151–3158.https://doi.org/10.1016/j.jssc.2006.06.011
Tamura, A., Takai, S., Yabutsuka, T., and Yao, T., Relaxation analysis of LixNiO2 and Lix(NCA)O2 in the deeply lithium extracted region (x ≤ 0.12), J. Electrochem. Soc., 2017, vol. 167, no. 7, pp. A1514–A1519.https://doi.org/10.1149/2.0691707jes
Kang, J., Takai, S., Yabutsuka, T., and Yao, T., Structural relaxation of Lix(Ni0.874Co0.090Al0.036)O2 after lithium extraction down to x = 0.12, Materials, 2018, vol. 11, no. 9, paper 1299.https://doi.org/10.3390/ma11081299
Konar, B., Van Ende, M.-A., and Jung, I.-H., Critical evaluation and thermodynamic optimization of the Li2O–Al2O3 and Li2O–MgO–Al2O3 systems, Metall. Mater. Trans. B, 2018, vol. 49, no. 7, pp. 2917–2944.https://doi.org/10.1007/s11663-018-1349-x
Gomes, K.Q., Bacelos, M., and Filho, P.I.P., Quantification of phase compositions in the system [(NiO)x] ∙ [(CoO)1 –x] ∙ [(Al2O3)], 0.05 ≤ x ≤ 0.95, and the effect in the electrical properties of semiconductor as a consequence of the CO+2 variation, Mater. Sci. Forum., 2012, vols. 727–728, pp. 550–555. https://doi.org/10./14028/www.scientific.net/MSF.727-728.550
Pan, D., Yuan, D., Sun, H., Duan, X., Luan, C., Guo, S., Li, Z., and Wang, L., Preparation and characterization of Co2+-doped LiAl5O8 nano-crystal powders by sol–gel technique, Mater. Chem. Phys., 2006, vol. 96, pp. 317–320.https://doi.org/10.1016/j.matchemphys.2005.07.020
Raj, C.J., Lincoln, M.B., and Das, S.J., Synthesis and characterization of doped lithium aluminate nanocrystalline particles by sol–gel method, Cryst. Res. Technol., 2008, vol. 43, no. 8, pp. 823–827.https://doi.org/10.1002/crat.200811165
Kaboon, S. and Hu, Y.H., Study of NiO–CoO and Co3O4–Ni3O4 solid solutions in multiphase Ni–Co–O systems, Ind. Eng. Chem. Res., 2011, vol. 50, no. 4, pp. 2015–2020.https://doi.org/10.1021/ie101249r
Li, Y., Han, X., Yi, T., He, Y., and Li, X., Review and prospect of NiCo2O4-based composite materials for supercapacitor electrodes, J. Energy Chem., 2019, vol. 31, pp. 54–78.https://doi.org/10.1016/j.jechem.2018.05.010
Peña, O., Bodenez, V., Guixouarn, T., Meza, E., and Gautier, J.L., Magnetic properties of lithium-based spinels Li(NixCo2 –x)O4, J. Magn. Magn. Mater., 2004, vols. 272–276, pp. E1579–1580.https://doi.org/10.1016/j.jmmm.2003.12.813
Antolini, E., LixNi1 –xO (0 < x ≤ 0.3) solid solutions: formation, structure and transport properties, Mater. Chem. Phys., 2003, vol. 82, no. 3, pp. 937–948.https://doi.org/10.1016/j.matchemphys.2003.08.006
Wu, Y., Pasero, D., McCabe, E.E., Matsushima, Y., and West, A.R., Formation of disordered and partially ordered LixCo1 –xO, J. Mater. Chem., 2009, vol. 19, no. 10, pp. 1443–1448.https://doi.org/10.1039/b816486j
Kazenas, E.K. and Tsvetkov, Yu.V., Termodinamika ispareniya oksidov (Thermodynamics of Oxide Vaporization), Moscow: URSS, 2015.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education: state research target for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences; basic research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Nipan, G.D., Kornilov, D.Y. Phase Equilibria in the Li2O–Al2O3–Ni–Co–O System. Inorg Mater 56, 809–814 (2020). https://doi.org/10.1134/S0020168520070110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520070110