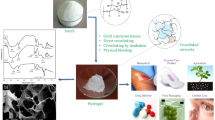
Abstract
Purpose
Among various types of external stimuli-responsive DDS, electric-responsive DDS are more promising carriers as they exploit less complex, easily miniaturized electric signal generators and the possibility of fine-tuning the electric signals. This study investigates the use of intrinsically biocompatible biopolymers in electro-simulative drug delivery to enhance the release of poorly-soluble/non-ionic drug.
Methods
CMC/PLA/ZnO/CUR nanocomposite films were prepared by the dispersion of CMC and ZnO NPs in solubilized PLA/curcumin medium, followed by solvent casting step. Curcumin is poorly water-soluble and used as the model drug in this study. The films with different contents of CMC, PLA and ZnO NPs were characterized using FTIR, impedance spectroscopy, tensile testing and FESEM imaging. The in vitro drug release of the films was carried out in deionized water under DC electric field of 4.5 V.
Results
The ionic conductivity of the films increased with increasing the CMC concentration of the film. The addition of a small amount of ZnO NPs (2%) successfully restored the tensile properties of the film. In response to the application of the electric field, the composite films released drug with a near-linear profile. There was no noticeable amount of passive diffusion of the drug from the film with the absence of the electric field.
Conclusion
The outcome of this study enabled the design of an electric-responsive nanocomposite platform for the delivery of poorly water-soluble/non-ionic drugs.














Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- ATR:
-
Attenuated total reflection
- CMC:
-
Carboxymethyl cellulose
- CPE:
-
Constant phase element
- CTC:
-
Charge transfer complexes
- CUR:
-
Curcumin
- DC:
-
Direct current
- DDS:
-
Drug delivery systems
- DEX:
-
Dexamethasone
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier-transform infrared spectroscopy
- GO:
-
Graphene oxide
- NP:
-
Nanoparticle
- PBS:
-
Phosphate-buffered saline
- PLA:
-
Poly(lactic acid)
- PPy:
-
Polypyrrole
- PVA:
-
Polyvinyl alcohol
- SGF:
-
Simulated gastric fluid
- UV:
-
Ultraviolet
- Zi:
-
Frequency dependence impedance properties- reactive part
- Zr:
-
Frequency dependence impedance properties-real part
References
Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–53.
Cui M, Shao Z, Lu D, Wang Y. Eco-friendly electrochemical biosensor based on sodium Carboxymethyl cellulose/reduced Graphene oxide composite. Macromol Res. 2019;27(4):327–33.
Nagesh M, Senthilkumar P, Jenifer Selvarani A, Raji P, Kasipandian K, Ponnaiah P, et al. Electricity generation using carboxymethyl cellulose and kitchen waste as substrate by Exiguobacterium sp SU-5 in mediatorless microbial fuel cell. J Pure Appl Microbiol. 2019;13(4):2151–8.
Knopf GK, Sinar D, Andrushchenko A, Nikumb S, editors. Flexible electrical circuits printed on polymers using graphene-cellulose inks. 2016 IEEE International Symposium on Circuits and Systems (ISCAS); 2016: IEEE.
Barras R, Cunha I, Gaspar D, Fortunato E, Martins R, Pereira L. Printable cellulose-based electroconductive composites for sensing elements in paper electronics. Flex Print Electron. 2017;2(1):014006.
Pyarasani RD, Jayaramudu T, John A. Polyaniline-based conducting hydrogels. J Mater Sci. 2019;54(2):974–96.
El-Sayed NS, Moussa MA, Kamel S, Turky G. Development of electrical conducting nanocomposite based on carboxymethyl cellulose hydrogel/silver nanoparticles@ polypyrrole. Synth Met. 2019;250:104–14.
Samsudin A, Isa M, editors. Conductivity and transport properties study of plasticized carboxymethyl cellulose (CMC) based solid biopolymer electrolytes (SBE). Advanced Materials Research; 2014: Trans Tech Publ.
Ramlli MA, Chai M, Isa M, editors. Influence of propylene carbonate as a plasticizer in CMC-OA based biopolymer electrolytes: conductivity and electrical study. Advanced Materials Research; 2013: Trans Tech Publ.
Chai M, Isa M. Novel proton conducting solid bio-polymer electrolytes based on carboxymethyl cellulose doped with oleic acid and plasticized with glycerol. Sci Rep. 2016;6(1):1–7.
Ramlli M, Maksud M, Isa M, editors. Characterization of polyethylene glycol plasticized carboxymethyl cellulose-ammonium fluoride solid biopolymer electrolytes. AIP Conference Proceedings; 2017: AIP Publishing LLC.
Rudhziah S, Ahmad A, Ahmad I, Mohamed N. Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochim Acta. 2015;175:162–8.
Saadiah M, Samsudin A, editors. Electrical study on Carboxymethyl Cellulose-Polyvinyl alcohol based bio-polymer blend electrolytes. IOP Conference Series: Materials Science and Engineering; 2018: IOP Publishing.
Isa M, Samsudin A. An enhancement on electrical properties of carboxymethyl cellulose-NH4Br based biopolymer electrolytes through impedance characterization. Int J Polym Anal Charact. 2017;22(5):447–54.
Hadi A, Hashim A. Development of a new humidity sensor based on (carboxymethyl cellulose–starch) blend with copper oxide nanoparticles. Ukr J Phys. 2017;62(12):1044.
Park CH, Kim DW, Prakash J, Sun Y-K. Electrochemical stability and conductivity enhancement of composite polymer electrolytes. Solid State Ionics. 2003;159(1–2):111–9.
Low S, Ahmad A, Hamzah H, Rahman MYA. Nanocomposite solid polymeric electrolyte of 49% poly (methyl methacrylate)-grafted natural rubber–titanium dioxide–lithium tetrafluoroborate (MG49-TiO 2-LiBF 4). J Solid State Electrochem. 2011;15(11–12):2611–8.
Zhao X, Clifford A, Poon R, Mathews R, Zhitomirsky I. Carboxymethyl cellulose and composite films prepared by electrophoretic deposition and liquid-liquid particle extraction. Colloid Polym Sci. 2018;296(5):927–34.
Holmes R, Wolfe SW. Effect of carboxymethylcellulose on the electrophoresis of serum proteins on paper. Arch Biochem Biophys. 1960;87(1):13–8.
Johnson G, Lewis K. Mechanism for the anti-redeposition action of sodium carboxy-methyl cellulose with cotton. II. Colloid-stability theory applied to the fibre-soil system. J Appl Chem. 1967;17(10):283–7.
Andonegi M, Peñalba M, de la Caba K, Guerrero P. ZnO nanoparticle-incorporated native collagen films with electro-conductive properties. Mater Sci Eng C. 2020;108:110394.
Yang Y, Ching YC, Chuah CH. Applications of lignocellulosic fibers and lignin in bioplastics: a review. Polymers. 2019;11(751). https://doi.org/10.3390/polym11050751.
Siddiqi KS, ur Rahman A, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):1–13.
Sampath U, Ching YC, Chuah CH, Sabariah J, Lin PC. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials. 2016;9(12):991.
Yi Y, Sun J, Lu Y, Liao Y. Programmable and on-demand drug release using electrical stimulation. Biomicrofluidics. 2015;9(2):022401
Udenni Gunathilake, Ching YC, Ching KY, Chuah CH, . Abdullah LC, Biomedical and microbiological applications of bio-based porous materials: a review. Polymers 2017. 9 (5): 160.
Sampath UTM, Ching YC, Chuah CH, Singh R, Lin PC. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose. 2017;24(5):2215–28.
Thennakoon MSUG, Ching YC, Chuah CH, Noorsaadah AR, Liou NS. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int J Biol Macromol. 2020;158:670–88.
Puiggalí-Jou A, Micheletti P, Estrany F, del Valle LJ, Alemán C. Electrostimulated release of neutral drugs from Polythiophene nanoparticles: smart regulation of drug–polymer interactions. Adv Healthc Mater. 2017;6(18):1700453.
Puiggalí-Jou A, Del Valle LJ, Alemán C. Cell responses to electrical pulse stimulation for anticancer drug release. Materials. 2019;12(16):2633.
Puiggalí-Jou A, Cejudo A, del Valle LJ, Alemán C. Smart drug delivery from electrospun fibers through electroresponsive polymeric nanoparticles. ACS Appl Bio Mater. 2018;1(5):1594–605.
Fu L-H, Qi C, Ma M-G, Wan P. Multifunctional cellulose-based hydrogels for biomedical applications. J Mater Chem B. 2019;7(10):1541–62.
Butun S, Ince FG, Erdugan H, Sahiner N. One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr Polym. 2011;86(2):636–43.
Veeramachineni AK, Sathasivam T, Muniyandy S, Janarthanan P, Langford SJ, Yan LY. Optimizing extraction of cellulose and synthesizing pharmaceutical grade carboxymethyl sago cellulose from malaysian sago pulp. Appl Sci. 2016;6(6):170.
Suntako R. Effect of zinc oxide nanoparticles synthesized by a precipitation method on mechanical and morphological properties of the CR foam. Bull Mater Sci. 2015;38(4):1033–8.
Mohammed NA, Habil NY. Evaluation of antimicrobial activity of curcumin against two oral bacteria. Autom Control Intell Syst. 2015;3:18–21.
Vitamin P. Formulation and physical characterization of microemulsions based carboxymethyl cellulose as vitamin C carrier. Malaysian J Anal Sci. 2015;19(1):275–83.
Rokesh K, Nithya A, Jeganathan K, Jothivenkatachalam K. A facile solid state synthesis of cone-like ZnO microstructure an efficient solar-light driven photocatalyst for rhodamine B degradation. Materials Today: Proceedings. 2016;3(10):4163–72.
de Peres ML, Delucis RA, Amico SC, Gatto DA. Zinc oxide nanoparticles from microwave-assisted solvothermal process: Photocatalytic performance and use for wood protection against xylophagous fungus. Nanomater Nanotechno. 2019;9:1847980419876201.
Manyasree D, Kiranmayi P, Venkata RK. Characterization and antibacterial activity of ZnO nanoparticles synthesized by co-precipitation method. Int J App Pharm. 2018;10(6):224–8.
Gunathilake TMSU, Ching YC, Chuah CH, Illias HA, Ching KY, Singh R, et al. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int J Biol Macromol. 2018;118:1055–64.
Kolev TM, Velcheva EA, Stamboliyska BA, Spiteller M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int J Quantum Chem. 2005;102(6):1069–79.
Bich VT, Thuy NT, Binh NT, Huong NTM, Yen PND, Luong TT. Structural and spectral properties of curcumin and metal-curcumin complex derived from turmeric (Curcuma longa). Physics and engineering of new materials: Springer. 2009:271–8.
Chieng B, Ibrahim N, Yunus W, Hussein M. Poly (lactic acid)/poly (ethylene glycol) polymer nanocomposites: effects of graphene nanoplatelets. Polymers. 2014;6(1):93–104.
Udeni Gunathilake T, Ching YC, Chuah CH. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers. 2017;9(2):64.
Abouzari MS, Berkemeier F, Schmitz G, Wilmer D. On the physical interpretation of constant phase elements. Solid State Ionics. 2009;180(14–16):922–7.
Wei S, Ching YC, Chuah CH. Synthesis of chitosan aerogels as promising carriers for drug delivery: a review. Carbohydr Polym. 2019;231:115744. https://doi.org/10.1016/j.carbpol.2019.115744.
Morsi M, Oraby A, Elshahawy A, El-Hady RA. Preparation, structural analysis, morphological investigation and electrical properties of gold nanoparticles filled polyvinyl alcohol/carboxymethyl cellulose blend. J Mater Res Technol. 2019;8(6):5996–6010.
Kale RD, Gorade VG, Madye N, Chaudhary B, Bangde PS, Dandekar PP. Preparation and characterization of biocomposite packaging film from poly (lactic acid) and acylated microcrystalline cellulose using rice bran oil. Int J Biol Macromol. 2018;118:1090–102.
H-x L, Zare Y, Rhee KY. The percolation threshold for tensile strength of polymer/CNT nanocomposites assuming filler network and interphase regions. Mater Chem Phys. 2018;207:76–83.
Razavi R, Zare Y, Rhee KY. A model for tensile strength of polymer/carbon nanotubes nanocomposites assuming the percolation of interphase regions. Colloids Surf Physicochem Eng Aspects. 2018;538:148–54.
Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):214.
Zare Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos Part A Appl Sci Manuf. 2016;84:158–64.
Zare Y. Modeling the strength and thickness of the interphase in polymer nanocomposite reinforced with spherical nanoparticles by a coupling methodology. J Colloid Interface Sci. 2016;465:342–6.
Luo N, Varaprasad K, Reddy GVS, Rajulu AV, Zhang J. Preparation and characterization of cellulose/curcumin composite films. RSC Adv. 2012;2(22):8483–8.
Baek SK, Song KB. Characterization of active biodegradable films based on proso millet starch and curcumin. Starch-Stärke. 2019;71(3–4):1800174.
Ngo TMP, Dang TMQ, Tran TX, Rachtanapun P. Effects of zinc oxide nanoparticles on the properties of pectin/alginate edible films. Int J Polym Sci. 2018;2018:1–9.
Fakhari S, Jamzad M, Kabiri FH. Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem Lett Rev. 2019;12(1):19–24.
Haniffa MACM, Hazlee AI, Ching YC, Shaliza I, Viorel S, Chuah CH. Nonisocyanate poly(hydroxyl urethane)-based green polymer hybrid coating systems: tailoring of biomacromolecular compound architecture using APTMS-ZnO/TEMPO-oxidized cellulose nanoparticles. ACS Omega. 2020;5(18):10315–26.
Salvador MD, Amigó V, Sahuquillo O, Antolinos CM, Segovia F, Vicente A, et al., editors. Thermal analysis of polymer resin matrix reinforced with E-glass fibers degradated in neutral environment. Proceedings of 11th European conference on composite materials; 2004.
Shinyama K. Mechanical and electrical properties of polylactic acid with aliphatic-aromatic polyester. J Eng. 2018;2018:1–7.
Qiao R, Deng H, Putz KW, Brinson LC. Effect of particle agglomeration and interphase on the glass transition temperature of polymer nanocomposites. J Polym Sci B Polym Phys. 2011;49(10):740–8.
Restrepo I, Benito N, Medinam C, Mangalaraja R, Flores P, Rodriguez-Llamazares S. Development and characterization of polyvinyl alcohol stabilized polylactic acid/ZnO nanocomposites. Mater Res Express. 2017;4(10):105019.
Kim I, Viswanathan K, Kasi G, Sadeghi K, Thanakkasaranee S, Seo J. Poly (lactic acid)/ZnO bionanocomposite films with positively charged ZnO as potential antimicrobial food packaging materials. Polymers. 2019;11(9):1427.
Aouat T, Kaci M, Devaux E, Campagne C, Cayla A, Dumazert L, et al. Morphological, mechanical, and thermal characterization of poly (lactic acid)/cellulose multifilament fibers prepared by melt spinning. Adv Polym Technol. 2018;37(4):1193–205.
Aouat T, Kaci M, Lopez-Cuesta J-M, Devaux E. Investigation on the durability of PLA Bionanocomposite fibers under hygrothermal conditions. Front Mater Sci. 2019;6:323–15.
Ching YC, Gunathilake TMS, Chuah CH, Ching KY, Singh R, Liou NS. Curcumin/tween 20-incorporated cellulose nanoparticles with enhanced curcumin solubility for nano-drug delivery: characterization and in vitro evaluation. Cellulose. 2019;26(9):5467–81.
Silvestre C, Cimmino S, Pezzuto M, Marra A, Ambrogi V, Dexpert-Ghys J, et al. Preparation and characterization of isotactic polypropylene/zinc oxide microcomposites with antibacterial activity. Polym J. 2013;45(9):938–45.
Ramos M, Fortunati E, Peltzer M, Jimenez A, Kenny JM, Garrigós MC. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym Degrad Stab. 2016;132:2–10.
Hu C, Li Y, Zhang N, Ding Y. Synthesis and characterization of a poly (o-anisidine)–SiC composite and its application for corrosion protection of steel. RSC Adv. 2017;7(19):11732–42.
Díez-Pascual AM, Díez-Vicente AL. Poly (3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int J Mol Sci. 2014;15(6):10950–73.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18.
Casalini T, Rossi F, Castrovinci A, Perale G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front Bioeng Biotech. 2019;7.
Swamy BY, Yun Y-S. In vitro release of metformin from iron (III) cross-linked alginate–carboxymethyl cellulose hydrogel beads. Int J Biol Macromol. 2015;77:114–9.
Kadry G. Comparison between gelatin/carboxymethyl cellulose and gelatin/carboxymethyl nanocellulose in tramadol drug loaded capsule. Heliyon. 2019;5(9):e02404.
Reich G. Ultrasound-induced degradation of PLA and PLGA during microsphere processing: influence of formulation variables. Eur J Pharm Biopharm. 1998;45(2):165–71.
Madusanka N, de Silva KN, Amaratunga G. A curcumin activated carboxymethyl cellulose–montmorillonite clay nanocomposite having enhanced curcumin release in aqueous media. Carbohydr Polym. 2015;134:695–9.
Ung VY, Foshaug RR, MacFarlane SM, Churchill TA, Doyle JS, Sydora BC, et al. Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Dig Dis Sci. 2010;55(5):1272–7.
Thennakoon MSU, Ching YC, Chuah CH, Noorsaadah AR, Liou NS. pH-responsive poly(lactic acid)/sodium carboxymethyl cellulose film for enhanced delivery of curcumin in vitro. Journal of Drug Delivery Science and Technology. 2020;58:101787.
Lopez CG, Colby RH, Cabral JT. Electrostatic and hydrophobic interactions in NaCMC aqueous solutions: effect of degree of substitution. Macromolecules. 2018;51(8):3165–75.
Wang C, Zheng Y. Oral 4-(N)-stearoyl gemcitabine nanoparticles inhibit tumor growth in mouse models. Oncotarget. 2017;8(52):89876–86.
Moussawi RN, Patra D. Nanoparticle self-assembled grain like curcumin conjugated ZnO: curcumin conjugation enhances removal of perylene, fluoranthene and chrysene by ZnO. Sci Rep. 2016;6(1):1–13.
Khosropanah MH, Dinarvand A, Nezhadhosseini A, Haghighi A, Hashemi S, Nirouzad F, et al. Analysis of the antiproliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iran J Pharm Res. 2016;15(1):231–9.
Koohpar ZK, Entezari M, Movafagh A, Hashemi M. Anticancer activity of curcumin on human breast adenocarcinoma: role of Mcl-1 gene. Iran J Cancer Prev. 2015;8(3).
Gardella L, Colonna S, Fina A, Monticelli O. A novel electrostimulated drug delivery system based on PLLA composites exploiting the multiple functions of graphite nanoplatelets. ACS Appl Mater Interfaces. 2016;8(37):24909–17.
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2011;4(8):1158–71.
Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10.
Dash A, Cudworth G II. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40(1):1–12.
Hetal T, Bindesh P, Sneha T. A review on techniques for oral bioavailability enhancement of drugs. Int J Pharm Sci Rev Res. 2010;4(3):033.
Shah P, Goodyear B, Michniak-Kohn BB. A review: enhancement of solubility and oral bioavailability of poorly soluble drugs. Adv Pharm J. 2017;2(5):161–73.
Stewart SA, Domínguez-Robles J, Donnelly RF, Larrañeta E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers. 2018;10(12):1379.
Grumezescu V, Grumezescu A. Materials for biomedical engineering: thermoset and thermoplastic polymers: Elsevier; 2019.
Song B, Zhao M, Forrester JV, McCaig CD. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci. 2002;99(21):13577–82.
Hess R, Jaeschke A, Neubert H, Hintze V, Moeller S, Schnabelrauch M, et al. Synergistic effect of defined artificial extracellular matrices and pulsed electric fields on osteogenic differentiation of human MSCs. Biomaterials. 2012;33(35):8975–85.
Antov Y, Barbul A, Mantsur H, Korenstein R. Electroendocytosis: exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys J. 2005;88(3):2206–23.
Teissie J, Rols M-P. An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys J. 1993;65(1):409–13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Poly(lactic acid)/carboxymethyl cellulose/ZnO/curcumin nanocomposite film was prepared using solvent casting method.
The emulsification and electrophoretic properties of carboxymethyl cellulose have been utilized for the electro-stimulated drug delivery process.
The study demonstrates the approach to trigger the release of poorly water-soluble and non-ionic drugs under electric stimulation.
The film does not show noticeable natural drug release providing that the total control of drug release under electrical stimulation.
The biopolymers provide an alternative for the use of nondegradable conductive polymers in electro-simulative drug delivery systems.
Rights and permissions
About this article
Cite this article
Gunathilake, T.M.S.U., Ching, Y.C., Chuah, C.H. et al. Electro-Stimulated Release of Poorly Water-Soluble Drug from Poly(Lactic Acid)/Carboxymethyl Cellulose/ZnO Nanocomposite Film. Pharm Res 37, 178 (2020). https://doi.org/10.1007/s11095-020-02910-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02910-z