Abstract
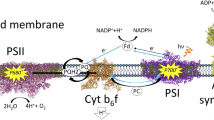
In memory of the 85th birthday of Yakov S. Lebedev (Moscow), who died in 1996, we start this Review on soft-glass matrix effects in donor–acceptor complexes with an appreciation of his pioneering work on high-field EPR spectroscopy on tribochemically generated donor–acceptor complexes. The mechanochemical activation of polycrystalline mixtures of porphyrins (and other donors) and quinone acceptors was found to produce large concentrations of triplet donor molecules and donor–acceptor radical pairs with unusual stability. The Review is continued with reporting on W-band high-field EPR and fast-laser studies on disaccharide matrix effects on structure and dynamics of donor–acceptor protein complexes related to photosynthesis, including the non-oxygenic bacterial reaction center (RC) and the oxygenic RCs Photosystem I (PS I) and Photosystem II (PS II, preliminary results). Some organisms can survive complete dehydration and high temperatures by adopting an anhydrobiotic state in which the intracellular medium contains large amounts of disaccharides, in particular trehalose and sucrose. Trehalose is most effective in protecting biostructures, both in vivo and in vitro. To clarify the molecular mechanisms of disaccharide bioprotection, structure and dynamics of sucrose and trehalose matrices at different controlled hydration levels were probed by perdeuterated nitroxide spin labels and native cofactor intermediates in their charge-separated states. Trehalose forms a homogeneous amorphous phase in which the hosted molecules are uniformly distributed. Notably, their rotational mobility at room temperature is dramatically impaired by the trehalose H-bonding network confinement to an extent that in normal protein–matrix systems is only observed at low temperatures around 150 K. From the experimental results, formation of an extended H-bonding network of trehalose with protein molecules is inferred, involving both bulk and local water molecules. The H-bond network extends homogeneously over the whole matrix integrating and immobilizing the hosted protein. Taken together, these observations suggest that in photosystems, such as bacterial RCs and PS I complexes, of different size and complexity regarding subunit composition and oligomeric organization, the molecular configuration of the cofactors involved in the primary processes of charge separation is not significantly distorted by incorporation into trehalose glass, even under extensive dehydration. By means of pulsed W-band high-field multiresonance EPR spectroscopies, such as ELDOR-detected NMR and ENDOR, in conjunction with using isotope labeled water (D2O and H217O), the biologically important issue of sensing and quantification of local water in proteins is addressed. The bacterial RC embedded into the trehalose glass matrix is used as model system. The two native radical cofactor ions of the primary electron donor and acceptor as well as an artificial nitroxide spin label site-specifically attached to the protein surface are studied in the experiments. The three paramagnetic reporter groups probe distinctly different local environments. They sense water molecules via their magnetic hyperfine and quadrupole interactions with either deuterons or 17O nuclei. It is shown that by using oxygen-17 labeled water, quantitative conclusions can be drawn differentiating between local and bulk water. It is concluded that dry trehalose operates as anhydrobiotic protein stabilizer by means of selective changes in the first solvation shell of the protein upon trehalose–matrix dehydration with subsequent changes in the hydrogen-bonding network. Such changes usually have an impact on the global function of a biological system. Finally, preliminary results of optical and W-band EPR experiments on the extremolytes ectoine and its derivative hydroxyectoine are reported; these compounds appear to share several stress-protecting properties with trehalose in terms of stabilizing protein matrices. For instance, they display remarkable stabilizing capabilities towards sensitive proteins and enzymes with respect to freeze-thawing, heat-treatment, and freeze-drying procedures. Moreover, hydroxyectoine is a good glass-forming compound and exhibits a remarkable bioprotective effect against desiccation and heat denaturation of functional protein complexes.























Similar content being viewed by others
References
Y.S. Lebedev, in Foundations of Modern EPR, ed. by G.R. Eaton, S.S. Eaton, K.M. Salikhov (World Scientific, Singapore, 1998), p. 731
O. Grinberg, L.J. Berliner (eds.), Very High Frequency (VHF) ESR/EPR (Springer, New York, 2004)
K. Möbius, A. Savitsky, High-Field EPR Spectroscopy on Proteins and Their Model Systems (RSC Publishing, Cambridge, 2009)
S.M. Hsu, J. Zhang, Z. Yin, Tribol. Lett. 13, 131 (2002)
K.-P. Müller, Lehrbuch der Oberflächentechnik (Vieweg, Braunschweig, 1996)
M. Scherge, S. Gorb, Biological Micro- and Nanotribology, Nature’s Solutions (Springer, Berlin, 2001)
X. He, S.H. Kim, Langmuir 33, 2717 (2017)
Y. Wang, N. Yamada, J. Xu, J. Zhang, Q. Chen, Y. Ootani, Y. Higuchi, N. Ozawa, M.-I. De Barros Bouchet, J.M. Martin, S. Mori, K. Adachi, M. Kubo, Sci. Adv. 5, eaax9301 (2019)
S.D. Chemerisov, O.Y. Grinberg, D.S. Tipikin, Y.S. Lebedev, H. Kurreck, K. Möbius, Chem. Phys. Lett. 218, 353 (1994)
D. Gust, T.A. Moore, Advan. Photochem. 16, 1 (1991)
J. von Gersdorff, M. Huber, H. Schubert, D. Niethammer, B. Kirste, M. Plato, K. Möbius, H. Kurreck, R. Eichberger, R. Kietzmann, F. Willig, Angew. Chem. Intern. Ed. Engl. 29, 670 (1990)
F. Lendzian, J. Schlüpmann, J. von Gersdorff, K. Möbius, H. Kurreck, Angew. Chem. Intern. Ed. Engl. 30, 1461 (1991)
M.R. Wasielewski, Chem. Rev. 92, 435 (1992)
F. Pöllinger, H. Heitele, M.E. Michel-Beyerle, C. Anders, M. Futscher, H.A. Staab, Chem. Phys. Lett. 198, 645 (1992)
K. Hasharoni, H. Levanon, J. von Gersdorff, H. Kurreck, K. Möbius, J. Chem. Phys. 98, 2916 (1993)
F. Lendzian, M. Huber, R.A. Isaacson, B. Endeward, M. Plato, B. Bönigk, K. Möbius, W. Lubitz, G. Feher, Biochim. Biophys. Acta 1183, 139 (1993)
T.F. Prisner, A. van der Est, R. Bittl, W. Lubitz, D. Stehlik, K. Möbius, Chem. Phys. 194, 361 (1995)
W. Lubitz, Phys. Chem. Chem. Phys. 4, 5539 (2002)
H. Kurreck, M. Huber, Angew. Chem. Intern. Ed. Engl. 34, 849 (1995)
D. Gust, T.A. Moore, A.L. Moore, Faraday Discuss. 155, 9 (2012)
T.A. Faunce, W. Lubitz, A.W. Rutherford, D. MacFarlane, G.F. Moore, P. Yang, D.G. Nocera, T.A. Moore, D.H. Gregory, S. Fukuzumi, K.B. Yoon, F.A. Armstrong, M.R. Wasielewski, S. Styring, Energy Environ. Sci. 6, 695 (2013)
D.R. Whang, D.H. Apaydin, Chem. Photo. Chem. 2, 148 (2018)
B. Zhang, L. Sun, Chem. Soc. Rev. 48, 2216 (2019)
G. Heinicke, Tribochemistry (Carl Hanser, Munich, 1984)
Y.S. Lebedev, in Modern Pulsed and Continuous-Wave Electron Spin Resonance, ed. by L. Kevan, M.K. Bowman (Wiley, New York, 1990), p. 365
N.M. Atherton, Electron Spin Resonance, Theory and Applications (Wiley, New York, 1973)
S.N. Dobryakov, G.G. Lazarev, M.V. Serdobov, Y.S. Lebedev, Mol. Phys. 36, 877 (1978)
C.A. Hutchison Jr., in The Triplet State, ed. by A.B. Zahlan (Cambridge University Press, Cambridge, 1967), p. 63
A.W. Hornig, J.S. Hyde, Mol. Phys. 6, 33 (1963)
R. Huber, M. Schwoerer, C. Bubeck, H. Six, Chem. Phys. Lett. 53, 35 (1978)
D.S. Tipikin, G.G. Lazarev, Y.S. Lebedev, Russian. J. Phys. Chem. 67, 159 (1993)
S.D. Chemerisov, G.D. Perekhodtsev, D.S. Tipikin, Ya.S. Lebedev, A.I. Prokofev, A.I. Aleksandrov, A.A. Dubinskii, K. Möbius, O.G. Poluektov, J. Schmidt, J. Chem. Soc., Faraday Trans. 92, 1959 (1996)
D.S. Tipikin, Y.S. Lebedev, O.G. Poluektov, J. Schmidt, Chem. Phys. Lett. 215, 199 (1993)
A.I. Aleksandrov, A.I. Prokofev, I. Yu Metlenkova, N.N. Bubnov, D.S. Tipikin, S.D. Chemerisov, G.D. Perekhodtsev, Y.S. Lebedev, Russ. J. Phys. Chem. 69, 743 (1995)
A.I. Aleksandrov, A.I. Prokofev, I. Yu Metlenkova, N.N. Bubnov, D.S. Tipikin, S.D. Chemerisov, G.D. Perekhodtsev, Y.S. Lebedev, Russ. J. Phys. Chem. 70, 25 (1996)
C.P. Poole Jr., Electron Spin Resonance (Wiley, New York, 1983)
R.T. Weber, J.A.J.M. Disselhorst, L.J. Prevo, J. Schmidt, WTh Wenckebach, J. Magn. Reson. 81, 129 (1989)
O. Burghaus, M. Rohrer, T. Götzinger, M. Plato, K. Möbius, Meas. Sci. Technol. 3, 765 (1992)
K. Möbius, M. Plato, W. Lubitz, Phys. Rev. 87, 172 (1992)
L. Cordone, G. Cottone, S. Giuffrida, G. Palazzo, G. Venturoli, C. Viappiani, Biochim. Biophys. Acta, Proteins Proteomics 1749, 252 (2005)
L. Cordone, G. Cottone, A. Cupane, A. Emanuele, S. Giuffrida, M. Levantino, Curr. Org. Chem. 19, 1684 (2015)
R.H. Austin, K.W. Beeson, L. Eisenstein, H. Frauenfelder, I.C. Gunsalus, Biochemistry 14, 5355 (1975)
R.H. Austin, A. Xie, L. van der Meer, B. Redlich, P.-A. Lindgård, H. Frauenfelder, D. Fu, Phys. Rev. Lett. 94, 128101 (2005)
D. Kleinfeld, M.Y. Okamura, G. Feher, Biochemistry 23, 5780 (1984)
B.H. McMahon, J.D. Muller, C.A. Wraight, G.U. Nienhaus, Biophys. J. 74, 2567 (1998)
P.R. Pokkuluri, P.D. Laible, A.E. Crawford, J.F. Mayfield, M.A. Yousef, S.L. Ginell, D.K. Hanson, M. Schiffer, FEBS Lett. 560, 171 (2004)
A. Savitsky, M. Malferrari, F. Francia, G. Venturoli, K. Möbius, J. Phys. Chem. B 114, 12729 (2010)
M. Malferrari, A. Savitsky, M.D. Mamedov, G.E. Milanovsky, W. Lubitz, K. Möbius, AYu Semenov, G. Venturoli, Biochim. Biophys. Acta 1857, 1440 (2016)
M. Malferrari, F. Francia, G. Venturoli, J. Phys. Chem. B 119, 13600 (2015)
S.J. Hagen, J. Hofrichter, W.A. Eaton, Science 269, 959 (1995)
S.J. Hagen, J. Hofrichter, W.A. Eaton, J. Phys. Chem. 100, 12008 (1996)
L. Cordone, P. Galajda, E. Vitrano, A. Gassmann, A. Ostermann, F. Parak, Eur. Biophys. J. 27, 173 (1998)
L. Cordone, M. Ferrand, E. Vitrano, G. Zaccai, Biophys. J. 76, 1043 (1999)
S.J. Clegg, Comp. Biochem. Physiol. B 128, 613 (2001)
L.M. Crowe, Comp. Biochem. Physiol. A 131, 505 (2002)
W.W. Parson, Biochim. Biophys. Acta 153, 248 (1968)
G. Feher, Photochem. Photobiol. 14, 373 (1971)
D. Stehlik, C.H. Bock, J. Petersen, J. Phys. Chem. 93, 1612 (1989)
A. Schnegg, M. Fuhs, M. Rohrer, W. Lubitz, T.F. Prisner, K. Möbius, J. Phys. Chem. B 106, 9454 (2002)
J.H. Freed, in Biological Magnetic Resonance, ed. by S.R. Eaton, G.R. Eaton, L.J. Berliner, vol. 24 (Kluwer, Boston, 2005) p. 239
M. Malferrari, F. Francia, G. Venturoli, J. Phys. Chem. B 115, 14732 (2011)
J.J. Max, C. Chapados, J. Phys. Chem. 116, 4626 (2002)
S. Giuffrida, G. Cottone, L. Cordone, Phys. Chem. Chem. Phys. 19, 4251 (2017)
P.K. Verma, A. Kundu, M.S. Puretz, C. Dhoonmoon, O.S. Chegwidden, C.H. Londergan, M. Cho, J. Phys. Chem. B 122, 2587 (2018)
J. Ingram, D. Bartels, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 377 (1996)
P. Alpert, Integr. Comp. Biol. 45, 685 (2005)
A. Tunnacliffe, J. Lapinski, Phil. Trans. R. Soc. Lond. B 358, 1755 (2003)
J.H. Crowe, J.F. Carpenter, L.M. Crowe, Annu. Rev. Physiol. 60, 73 (1998)
M. Sakurai, T. Furuki, K.-I. Akao, D. Tanaka, Y. Nakahara, T. Kikawada, M. Watanabe, T. Okuda, Proc. Natl. Acad. Sci. USA 105, 5093 (2008)
S.C. Hand, M.A. Menze, M. Toner, L. Boswell, D. Moore, Annu. Rev. Physiol. 73, 115 (2011)
T.C. Boothby, H. Tapia, A.H. Brozena, S. Piszkiewicz, A.E. Smith, A. Giovannini, L. Rebecchi, G.J. Pielak, D. Koshland, B. Goldstein, Mol. Cell 65, 975 (2017)
A. Eroglu, M.J. Russo, R. Bieganski, A. Fowler, S. Cheley, H. Bayley, M. Toner, 4 Nat. Biotechnol. 18, 163 (2000)
S. Ohtake, Y.J. Wang, J. Pharm. Sci. 100, 2020 (2011)
D.S. Dimitrov, Methods Mol. Biol. 899, 1 (2012)
M.C. Manning, D.K. Chou, B.M. Murphy, R.W. Payne, D.S. Katayama, Pharm. Res. 27, 544 (2010)
M.A. Mensink, H.W. Frijlink, K. van der Voort Maarschalk, W.L.J. Hinrichs, Eur. J. Pharm. Biopharm. 114, 288 (2017)
S. Giuffrida, G. Cottone, F. Librizzi, L. Cordone, J. Phys. Chem. B 107, 13211 (2003)
G. Caliskan, A. Kisliuk, A.M. Tsai, C.L. Soles, A.P. Sokolov, J. Phys. Chem. 118, 4230 (2003)
G. Caliskan, D. Mechtani, J.H. Roh, A. Kisliuk, A.P. Sokolov, S. Azzam, M.T. Cicerone, S. Lin-Gibson, I. Peral, J. Phys. Chem. 121, 1978 (2004)
A. Longo, S. Giuffrida, G. Cottone, L. Cordone, Phys. Chem. Chem. Phys. 12, 6852 (2010)
G. Bellavia, S. Giuffrida, G. Cottone, A. Cupane, L. Cordone, J. Phys. Chem. B. 115, 6340 (2011)
E.F. Semeraro, S. Giuffrida, G. Cottone, A. Cupane, J. Phys. Chem. B 121, 8731 (2017)
G. Cottone, L. Cordone, G. Ciccotti, Biophys. J. 80, 931 (2001)
G. Cottone, S. Giuffrida, G. Ciccotti, L. Cordone, Proteins: Struct., Funct., Bioinf. 59, 291 (2005)
A. Lerbret, F. Affouard, A. Hedoux, S. Krenzlin, J. Siepmann, M.-C. Bellissent-Funel, M. Descamps, J. Phys. Chem. B 116, 11103 (2012)
H. Frauenfelder, B.H. McMahon, Ann. Phys. (Leipzig) 9, 655 (2000)
J.G. Sampedro, S. Uribe, Mol. Cell. Biochem. 256, 319 (2004)
J.L. Green, C.A. Angell, J. Phys. Chem. 93, 2880 (1989)
C. Schebor, M.P. Buera, J. Chirife, J. Food. Eng. 30, 269 (1996)
B.S. Chang, R.M. Beauvais, A. Dong, J.F. Carpenter, Arch. Biochem. Biophys. 331, 249 (1996)
W.Q. Sun, P. Davidson, Biochim. Biophys. Acta 1425, 235 (1998)
M.T. Cicerone, J.F. Douglas, Soft Matter 8, 2983 (2012)
J. Buitink, C. Walters-Vertucci, F.A. Hoekstra, O. Leprince, Plant Physiol. 111, 235 (1996)
J.F. Carpenter, J.H. Crowe, Biochemistry 28, 3916 (1989)
S.N. Timasheff, Biochemistry 41, 13473 (2002)
T. Arakawa, S.N. Timasheff, Biochemistry 21, 6536 (1982)
P.S. Belton, A.M. Gill, Biopolymers 34, 957 (1994)
G. Cottone, G. Ciccotti, L. Cordone, J. Chem. Phys. 117, 9862 (2002)
C. Olsson, S. Genheden, V. García Sakai, J. Swenson, J. Phys. Chem. B 123, 3679 (2019)
S.N. Timasheff, Biochemistry 31, 9857 (1992)
T. Arakawa, S.N. Timasheff, Biophys. J. 47, 411 (1985)
F. Francia, M. Dezi, A. Mallardi, G. Palazzo, L. Cordone, G. Venturoli, J. Am. Chem. Soc. 130, 10240 (2008)
G. Cottone, S. Giuffrida, S. Bettati, S. Bruno, B. Campanini, M. Marchetti, S. Abbruzzetti, C. Viappiani, A. Cupane, A. Mozzarelli, L. Ronda, Catalists 9, 1024 (2019)
K.D. Rector, J. Jiang, M.A. Berg, M.D. Fayer, J. Phys. Chem. B. 105, 1081 (2001)
M. Tarek, D.J. Tobias, Phys. Rev. Lett. 88, 138101 (2002)
K. Wood, F.-X. Gallat, R. Otten, A.J. van Heel, M. Lethier, L. van Eijck, M. Moulin, M. Haertlein, M. Weik, F.A.A. Mulder, Angew. Chem. Int. Ed. 52, 665 (2013)
H. Frauenfelder, B.H. McMahon, Proc. Natl. Acad. Sci. USA 95, 4795 (1998)
F. Librizzi, C. Viappiani, S. Abbruzzetti, L. Cordone, J. Chem. Phys. 116, 1193 (2002)
A.M. Massari, I.J. Finkelstein, B.L. McClain, A. Goj, X. Wen, K.L. Bren, R.F. Loring, M.D. Faye, J. Am. Chem. Soc. 127, 14279 (2005)
M. Malferrari, A. Savitsky, W. Lubitz, K. Möbius, G. Venturoli, J. Phys. Chem. Lett. 7, 4871 (2016)
G. Palazzo, A. Mallardi, A. Hochkoeppler, L. Cordone, G. Venturoli, Biophys. J. 82, 558 (2002)
F. Francia, G. Palazzo, A. Mallardi, L. Cordone, G. Venturoli, Biophys. J. 85, 2760 (2003)
F. Francia, G. Palazzo, A. Mallardi, L. Cordone, G. Venturoli, Biochim. Biophys. Acta Bioenerg. 1658, 50 (2004)
P. Lunkenheimer, A. Loidl, Chem. Phys. 284, 205 (2002)
M. Orrit, Angew. Chem. Inter. Ed. 52, 163 (2013)
G.P. Johari, M. Goldstein, J. Chem. Phys. 55, 4245 (1971)
P. Allegrini, J.F. Douglas, S.C. Glotzer, Phys. Rev. E. 60, 5714 (1999)
K. Kaminski, E. Kaminska, K.L. Ngai, M. Paluch, P. Wlodarczyk, A. Kasprzycka, W. Szeja, J. Phys. Chem. B 113, 10088 (2009)
H. Frauenfelder, G. Chen, J. Berendzen, P.W. Fenimore, H. Jansson, B.H. McMahon, I.R. Stroe, J. Swenson, R.D. Young, Proc. Natl. Acad. Sci. USA 106, 5129 (2009)
M.D. Ediger, Ann. Rev. Phys. Chem. 51, 99 (2000)
R.A. Riggleman, J.F. Douglas, J.J. de Pablo, Soft Matter 6, 292 (2010)
L. Sloten, Biochim. Biophys. Acta 275, 208 (1972)
J.H. Freed, in Very High Frequency (VHF) ESR/EPR, ed. by O. Grinberg, L.J. Berliner (Springer, New York, 2004), p. 19
K. Möbius, W. Lubitz, N. Cox, A. Savitsky, Magnetochemistry 4, 50 (2018)
T.F. Prisner, M. Rohrer, K. Möbius, Appl. Magn. Reson. 7, 167 (1994)
K. Möbius, A. Savitsky, A. Schnegg, M. Plato, M. Fuchs, Phys. Chem. Chem. Phys. 7, 19 (2005)
A. Savitsky, J. Niklas, J.H. Golbeck, K. Möbius, W. Lubitz, Phys. Chem B. 117, 11184 (2013)
A. Nalepa, K. Möbius, W. Lubitz, A. Savitsky, J. Magn. Reson. 242, 203 (2014)
K. Möbius, W. Lubitz, A. Savitsky, Prog. Nucl. Magn. Res. Spec. 75, 1 (2013)
A. Schweiger, G. Jeschke, Principles of Pulse Electron Paramagnetic Resonance (Oxford University Press, Oxford, 2001)
D. Goldfarb, S. Stoll (eds.), EPR Spectroscopy: Fundamentals and Methods (Wiley, New York, 2018)
C. Kirmaier, D. Holten, Photosynth. Res. 13, 225 (1987)
G. Feher, J.P. Allen, M.Y. Okamura, D.C. Rees, Nature 339, 111 (1989)
J.C. Williams, R.G. Alden, H.A. Murchison, J.M. Peloquin, N.W. Woodbury, J.P. Allen, Biochemistry 31, 11029 (1992)
C.-K. Tang, J.A. Williams, A.K.W. Taguchi, P. James, J.P. Allen, N.W. Woodbury, Biochemistry 38, 8794 (1999)
M.Y. Okamura, M.L. Paddock, M.S. Graige, G. Feher, Biochim. Biophys. Acta 1458, 148 (2000)
H. Frauenfelder, B.H. McMahon, P.W. Fenimore, Proc. Natl. Acad. Sci. USA 100, 8615 (2003)
H. Wang, S. Lin, J.P. Allen, J.C. Williams, S. Blankert, C. Laser, N.W. Woodbury, Science 316, 747 (2007)
G. Katona, A. Snijder, P. Gourdon, U. Andréasson, Ö. Hansson, L.-E. Andréasson, R. Neutze, Nat. Struct. Mol. Biol. 12, 630 (2005)
M.H.B. Stowell, T.M. McPhillips, D.C. Rees, S.M. Soltis, E. Abresch, G. Feher, Science 276, 812 (1997)
Q. Xu, M.R. Gunner, Biochemistry 40, 3232 (2001)
Q. Xu, M.R. Gunner, Biochemistry 41, 2694 (2002)
Q. Xu, L. Baciou, P. Sebban, M.R. Gunner, Biochemistry 41, 10021 (2002)
J. Breton, C. Boullais, C. Mioskowski, P. Sebban, L. Baciou, E. Nabedryk, Biochemistry 41, 12921 (2002)
J. Breton, Biochemistry 43, 3318 (2004)
C.R.D. Lancaster, Biochim. Biophys. Acta 1365, 143 (1998)
A. Kuglstatter, U. Ermler, H. Michel, L. Baciou, G. Fritzsch, Biochemistry 40, 4253 (2001)
U. Zachariae, C.R.D. Lancaster, Biochim. Biophys. Acta 1505, 280 (2001)
S.E. Walden, R.A. Wheeler, J. Phys. Chem. B 106, 3001 (2002)
J.M. Kriegl, G.U. Nienhaus, Proc. Natl. Acad. Sci. USA 101, 123 (2004)
J.M. Kriegl, F.K. Forster, G.U. Nienhaus, Biophys. J. 85, 1851 (2003)
M. Malferrari, A. Savitsky, F. Francia, K. Möbius, G. Venturoli, in preparation (2020)
R.A. Marcus, N. Sutin, Biochim. Biophys. Acta Rev. Bioenerget. 811, 265 (1985)
C.C. Moser, J.M. Keske, K. Warncke, R.S. Farid, P.L. Dutton, Nature 355, 796 (1992)
S.S. Deshmukh, J.C. Williams, J.P. Allen, L. Kalman, Biochemistry 50, 340 (2011)
S.S. Deshmukh, J.C. Williams, J.P. Allen, L. Kalman, Biochemistry 50, 3321 (2011)
E. Nabedryk, K.A. Bagley, D.L. Thibodeau, M. Bauscher, W. Mäntele, J. Breton, FEBS Lett. 266, 59 (1990)
T. Iwata, M.L. Paddock, M.Y. Okamura, H. Kandori, Biochemistry 48, 1220 (2009)
M. Malferrari, A. Mezzetti, F. Francia, G. Venturoli, Biochim. Biophys. Acta Bioenerg. 1827, 328 (2013)
E.C. López-Díez, S. Bone, Biochim. Biophys. Acta 1673, 139 (2004)
B. Roser, Biopharm. 4, 47 (1991)
C. Colaco, S. Sen, M. Thangavelu, S. Pinder, B. Roser, Biotechnology 10, 1007 (1992)
M. Uritani, M. Takai, K. Yoshinaga, J. Biochem. 117, 774 (1995)
S. Giuffrida, G. Cottone, L. Cordone, J. Phys. Chem. B 108, 15415 (2004)
S. Giuffrida, G. Cottone, L. Cordone, Biophys. J. 91, 968 (2006)
M. Malferrari, A. Nalepa, G. Venturoli, F. Francia, W. Lubitz, K. Möbius, A. Savitsky, Phys. Chem. Chem. Phys. 16, 9831 (2014)
P. Gast, R.T.L. Herbonnet, J. Klare, A. Nalepa, C. Rickert, D. Stellinga, L. Urban, K. Möbius, A. Savitsky, H.J. Steinhoff, E.J.J. Groenen, Phys. Chem. Chem. Phys.16, 15910 (2014)
C.J. Roberts, P.G. Debenedetti, J. Phys. Chem. B 103, 7308 (1999)
P.B. Conrad, J.J. de Pablo, J. Phys. Chem. A 103, 4049 (1999)
A. Lerbret, P. Bordat, F. Affouard, M. Descamps, F. Migliardo, J. Phys. Chem. B 109, 11046 (2005)
G.A. Jeffrey, S. Takagi, Acc. Chem. Res. 11, 264 (1978)
D.B. Davies, J.C. Christofides, Carbohydr. Res. 163, 269 (1987)
N.C. Ekdawi-Sever, P.B. Conrad, J.J. de Pablo, J. Phys. Chem. A 105, 734 (2001)
S. Magazu, V. Villari, P. Migliardo, G. Maisano, M.T.F. Telling, J. Phys. Chem. B 105, 1851 (2001)
U. Heugen, G. Schwaab, E. Bruendermann, M. Heyden, X. Yu, D.M. Leitner, M. Havenith, Proc. Natl. Acad. Sci. USA 103, 12301 (2006)
F. Affouard, P. Bordat, M. Descamps, A. Lerbret, S. Magazu, F. Migliardo, A.J. Ramirez-Cuesta, M.F.T. Telling, Chem. Phys. 317, 258 (2005)
M.V. Fedorov, J.M. Goodman, D. Nerukh, S. Schumm, Phys. Chem. Chem. Phys. 13, 2294 (2011)
J.H. Golbeck (ed.), Photosystem I. The light-Driven Plastocyanin: Ferredoxin Oxidoreductase (Springer, Dordrecht, 2006)
P. Jordan, P. Fromme, H.T. Witt, O. Klukas, W. Saenger, N. Krauß, Nature 411, 909 (2001)
K. Brettel, W. Leibl, Biochim. Biophys. Acta 1507, 100 (2001)
M. Mamedov, Govindjee, V. Nadtochenko, A. Semenov, Photosynth. Res. 125, 51 (2015)
M. Guergova-Kuras, B. Boudreaux, A. Joliot, P. Joliot, K. Redding, Proc. Natl. Acad. Sci. USA 98, 4437 (2001)
N. Srinivasan, J.H. Golbeck, Biochim. Biophys. Acta 1787, 1057 (2009)
J. Deisenhofer, O. Epp, K. Miki, R. Huber, H. Michel, Nature 318, 618 (1985)
H. Komiya, T.O. Yeates, D.C. Rees, J.P. Allen, G. Feher, Proc. Natl. Acad. Sci. USA 85, 9012 (1988)
I.V. Shelaev, F.E. Gostev, M.D. Mamedov, O.M. Sarkisov, V.A. Nadtochenko, V.A. Shuvalov, A.Y. Semenov, Biochim. Biophys. Acta 1797, 1410 (2010)
K. Brettel, Biochim. Biophys. Acta 1318, 322 (1997)
P. Setif, H. Bottin, Biochemistry 28, 2689 (1989)
I.R. Vassiliev, Y.S. Jung, M.D. Mamedov, AYu Semenov, J.H. Golbeck, Biophys. J. 72, 301 (1997)
K. Brettel, J.H. Golbeck, Photosynth. Res. 45, 183 (1995)
D.A. Cherepanov, G.E. Milanovsky, O.A. Gopta, R. Balasubramanian, D.A. Bryant, A.Y. Semenov, J.H. Golbeck, J. Phys. Chem. B 122, 7943 (2018)
P. Sétif, P. Mathis, T. Vänngård, Biochim. Biophys. Acta 767, 404 (1984)
E. Schlodder, K. Falkenberg, M. Gergeleit, K. Brettel, Biochemistry 37, 9466 (1998)
G. Milanovsky, O. Gopta, A. Petrova, M. Mamedov, M. Gorka, D. Cherepanov, J. Golbeck, A. Semenov, Biochim. Biophys. Acta Bioenerget. 1860, 601 (2019)
I. Shelaev, M. Gorka, A. Savitsky, V. Kurashov, M. Mamedov, F. Gostev, K. Möbius, V. Nadtochenko, J. Golbeck, A. Semenov, Zeitschrift für Physikalische Chemie (J. Phys. Chem.) 231, 325 (2017)
V. Kurashov, M. Gorka, G.E. Milanovsky, T.W. Johnson, D.A. Cherepanov, AYu Semenov, J.H. Golbeck, Biochim. Biophys. Acta Bioenerget. 1859, 1288 (2018)
P. Fromme, I. Grotjohann, in Photosystem I. The Light-Driven Plastocyanin:Ferredoxin Oxidoreductase, ed. by J. Golbeck (Springer, Dordrecht, 2006), p. 47
V.P. Shinkarev, in Photosystem I. The Light-Driven Plastocyanin: Ferredoxin Oxidoreductase, ed. by J. Golbeck (Springer, Dordrecht, 2006), p. 612
S. Santabarbara, R. Jennings, G. Zucchelli, in The Biophysics of Photosynthesis, ed. by J. Golbeck, A. van der Est (Springer, New York, 2014) p. 241
D.H. Rasmussen, A.P. MacKenzie, J. Phys. Chem. 75, 967 (1971)
J.G. Constantin, M. Schneider, H.R. Corti, J. Phys. Chem. B 120, 5047 (2016)
P.J. Hore, in Advanced EPR. Applications in Biology and Biochemistry, ed. by A.J. Hoff (Elsevier, Amsterdam, 1989), p.405
M. Kanduč, A. Schlaich, E. Schneck, R.R. Netz, Langmuir 32, 8767 (2016)
C. Olsson, H. Jansson, J. Swenson, J. Phys. Chem. B 120, 4723 (2016)
A. Nalepa, M. Malferrari, W. Lubitz, G. Venturoli, K. Möbius, A. Savitsky, Phys. Chem. Chem. Phys. 19, 28388 (2017)
J. Koepke, E.M. Krammer, A.R. Klingen, P. Sebban, G.M. Ullmann, G. Fritzsch, J. Mol. Biol. 371, 396 (2007)
D.E. Giangiacomo, M.R. Gunner, L.P. Dutton, in Progress in Photosynthesis Research, ed. by J. Biggins (Springer, Dordrecht, 1987), p. 409
J.A. Rard, J. Solution Chem. 48, 271 (2019)
M. Malferrari, G. Venturoli, F. Francia, A. Mezzetti, Spectrosc. Int. J. 27, 337 (2012)
L.B. Rockland, Anal. Chem. 32, 1375 (1960)
S. Khodadadi, A.P. Sokolov, Biochim. Biophys. Acta 1861, 3546 (2017)
K. Henzler-Wildman, D. Kern, Nature 450, 964 (2007)
S. Khodadadi, A.P. Sokolov, Soft Matter 11, 4984 (2015)
R.G. Bryant, C. R. Phys. 11, 128 (2010)
B. Halle, Philos. Trans. R. Soc. Lond., B, Biol. Sci. 359, 1207 (2004)
N.V. Nucci, M.S. Pometun, A.J. Wand, J. Am. Chem. Soc. 133, 12326 (2011)
N.V. Nucci, M.S. Pometun, A.J. Wand, Nat. Struct. Mol. Biol. 18, 245 (2011)
K. Yokoyama, T. Kamei, H. Minami, M. Suzuki, J. Phys. Chem. B 105, 12622 (2001)
V.C. Nibali, M. Havenith, J. Am. Chem. Soc. 136, 12800 (2014)
A.I. McIntosh, B. Yang, S.M. Goldup, M. Watkinson, R.S. Donnan, Chem. Soc. Rev. 41, 2072 (2012)
A.C. Fogarty, D. Laage, J. Phys. Chem. B 118, 7715 (2014)
J.T. King, K.J. Kubarych, J. Am. Chem. Soc. 134, 18705 (2012)
D. Laage, G. Stirnemann, F. Sterpone, R. Rey, J.T. Hynes, Ann. Rev. Phys. Chem. 62, 395 (2011)
D.M. Leitner, M. Gruebele, M. Havenith, Hfsp J. 2, 314 (2008)
H.J. Bakker, J.L. Skinner, Chem. Rev. 110, 1498 (2010)
H. Frauenfelder, S.G. Sligar, P.G. Wolynes, Science 254, 1598 (1991)
H. Frauenfelder, P.W. Fenimore, G. Chen, B.H. McMahon, Proc. Nat. Acad. Sci. USA 103, 15469 (2006)
F. Sussich, C. Skopec, J. Brady, A. Cesaro, Carbohydr. Res. 334, 165 (2001)
W.L. Hubbell, in Membrane Protein Structure: Experimental Approaches, ed. by S.H. White (Oxford University Press, London, 1994), p. 224
P. Gajula, I.V. Borovykh, C. Beier, T. Shkuropatova, P. Gast, H.J. Steinhoff, Appl. Magn. Reson. 31, 167 (2007)
P. Ball, Chem. Rev. 108, 74 (2008)
P. Ball, Proc. Natl. Acad. Sci. USA 114, 13327 (2017)
A. Losi, W. Gärtner, Annu. Rev. Plant Biol. 63, 49–72 (2012)
V.P. Shinkarev, C.A. Wraight, Proc. Natl. Acad. Sci. USA 90, 1834 (1993)
F. Müh, C. Glöckner, J. Hellmich, A. Zouni, Biochim. Biophys. Acta 1817, 44 (2012)
J-R. Shen, Annu. Rev. Plant Biol. 66, 23 (2015)
F. Rappaport, B.A. Diner, Coord. Chem. Rev. 252, 259 (2008)
D.J. Vinyard, G.W. Brudvig, Annu. Rev. Phys. Chem. 68, 101 (2017)
N. Cox, D.A. Pantazis, W. Lubitz, Annu. Rev. Biochem. 89, 795 (2020)
Y. Umena, K. Kawakami, J.-R. Shen, N. Kamiya, Nature 473, 55 (2011)
W. Lubitz, M. Chrysina, N. Cox, Photosyn. Res. 142, 105 (2019)
L. Rapatskiy, N. Cox, A. Savitsky, W.M. Ames, J. Sander, M.M. Nowaczyk, M. Roegner, A. Boussac, F. Neese, J. Messinger, W. Lubitz, J. Am. Chem. Soc. 134, 16619 (2012)
K. Brettel, E. Schlodder, H.T. Witt, Biochim. Biophys. Acta 766, 403 (1984)
M. Karge, K.D. Irrgang, S. Sellin, R. Feinäugle, B. Liu, H.J. Eckert, H.J. Eichler, G. Renger, FEBS Lett. 378, 140 (1996)
C. Tommos, G.T. Babcock, Biochim. Biophys. Acta 1458, 199 (2000)
H. Conjeaud, P. Mathis, Biochim. Biophys. Acta 590, 353 (1980)
E. Schlodder, B. Meyer, Biochim. Biophys. Acta 890, 23 (1987)
E. Schlodder, M. Çetin, F. Lendzian, Biochim. Biophys. Acta 1847, 1283 (2015)
M.J. Schilstra, F. Rappaport, J.H.A. Nugent, C.J. Barnett, D.R. Klug, Biochemistry 37, 3974 (1998)
F. Rappaport, J. Lavergne, Biochim. Biophys. Acta 1503, 246 (2001)
M. Mamedov, F. Francia, L. Vitukhnovskaya, A. Semenov, G. Venturoli, in Proceedings of the 10th International Conference “Photosynthesis and Hydrogen Energy Research for Sustainability” (St. Petersburg, Russia, 23-28 June 2019) p. 75
B.A. Diner, D.A. Force, D.W. Randall, R.D. Britt, Biochemistry 37, 17931 (1998)
R. Ahlbrink, M. Haumann, D. Cherepanov, O. Bögershausen, A. Mulkidjanian, W. Junge, Biochemistry 37, 1131 (1998)
P.J. Steinbach, R. Ionescu, C.R. Matthews, Biophys. J. 82, 2244 (2002)
M. Mamedov, A. Semenov, F. Francia, G. Venturoli, in preparation (2020)
S. Reinmann, P. Mathis, Biochim. Biophys. Acta 635, 249 (1981)
S. Gerken, J.P. Dekker, E. Schlodder, H.T. Witt, Biochim. Biophys. Acta 977, 52 (1989)
B. Hillmann, E. Schlodder, Biochim. Biophys. Acta 1231, 76 (1995)
G. Lentzen, T. Schwarz, Appl. Microbiol. Biotechnol. 72, 623 (2006)
C.G. Hounsa, V.E. Brandt, J. Thevelein, S. Hohmann, B.A. Prior, Microbiology 144, 671 (1998)
P. Lamosa, L.O. Martins, M.S. da Costa, H. Santos, Appl. Environ. Microbiol. 6, 3591 (1998)
Z. Silva, S. Alarico, A. Nobre, R. Horlacher, J. Marugg, W. Boos, A.I. Mingote, M.S. da Costa, J. Bacteriol. 185, 5943 (2003)
C. De Virgilio, T. Hottinger, J. Domínguez, T. Boller, A. Wiemken, Eur. J. Biochem. 219, 179 (1994)
M.R. Michaud, D.L. Denlinger, J. Comp. Physiol. B 177, 753 (2007)
A. Kraegeloh, H.J. Kunte, Extremophiles 6, 453 (2002)
K. Lippert, E.A. Galinski, Appl. Microbiol. Biotechnol. 37, 61 (1992)
C. Tanne, E.A. Golovina, F.A. Hoekstra, A. Meffert, E.A. Galinski, Front. Microbiol. 5, 150 (2014)
I. Yu, M. Nagaoka, Chem. Phys. Lett. 388, 316 (2004)
G. Zaccai, I. Bgyan, J. Combet, G.J. Cuello, B. Demé, Y. Fichou, F.-X. Gallat, V.M. Galvan Josa, S. von Gronau, M. Haertlein, A. Martel, M. Moulin, M. Neumann, M. Weik, D. Oesterhelt, Sci. Rep. 6, 31434 (2016)
N. Borges, A. Ramos, N.D.H. Raven, R.J. Sharp, H. Santos, Extremophiles 6, 209 (2002)
A. Nalepa, K. Möbius, M. Plato, W. Lubitz, A. Savitsky, Appl. Magn. Reson. 50, 1 (2019)
Acknowledgements
Financial support from MIUR of Italy (RFO2018) is gratefully acknowledged by F. F. and G. V. This work was supported by the Russian Foundation for Basic Research (Grant 17-00-00201 to A. Yu. S.). The Max-Planck-Gesellschaft and the Cluster of Excellence RESOLV (EXC 1069), funded by the Deutsche Forschungsgemeinschaft (DFG), supported this work. K. M. gratefully acknowledges sustaining support by the Free University Berlin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Möbius, K., Savitsky, A., Malferrari, M. et al. Soft Dynamic Confinement of Membrane Proteins by Dehydrated Trehalose Matrices: High-Field EPR and Fast-Laser Studies. Appl Magn Reson 51, 773–850 (2020). https://doi.org/10.1007/s00723-020-01240-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-020-01240-y