Abstract
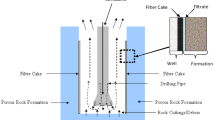
After drilling oil wells using oil-based drilling fluid, there is the occurrence of a formation of a thin layer of solids adsorbed on the surface, called filter cake, which must be removed to obtain satisfactory well cementation. The present work developed microemulsion systems from sodium dodecyl sulfate, a biodegradable surfactant, with kerosene as oil phase, n-butanol as cosurfactant and distilled water, in order to evaluate its efficiency in removing the filter cake. The results showed that the microemulsion systems composed of inverse and bicontinuous micelles removed 100% of the filter cake regardless of the content of the constituents in the formulation, while the direct micelles only reached 100% with the formulation composed by 10% kerosene and cosurfactant/surfactant ratio = 4. It was observed that the cosurfactant/surfactant ratio is a determining variable for direct micelles to remove 100% of the filter cake and that they are compatible with cement, offering desirable thermal stability and improving the wettability of the rock in water.
Similar content being viewed by others
Introduction
Oil well drilling activities take place with the help of drilling fluids, of which among several functions, one is the formation of a low permeability film on the rock with the function of reducing the loss of the fluid filtrate (Bageri et al. 2017). This film is called filter cake before the cementing operation begins, and the filter cake must be removed because the cement slurry loses its characteristics when contaminated with the constituents of the filter cake. This mixture causes the cement's hydration power to decrease, resulting in loss of compressive forces, shear and reduction of the setting time (Aughenbaugh et al. 2014; Li et al. 2015).
Removal of filter cake has been a challenge to the oil industry. Therefore, new technologies have been in research such as obtaining chemical formulations that can effectively remove the filter cake and not cause damages to the rock formation. Among these new technologies, the formulation of microemulsion systems has been highlighted, since it has the ability to remove filter cake with high efficiency without causing damages to the rock formation (Quintero et al. 2007, 2013).
The use of microemulsions as flushing fluid stands out since they are spontaneously obtained and present characteristics such as low interfacial tension, good solubilization capacity and high wettability in rocky surface (Schramm 2000; Dantas et al. 2001, 2019; Fanun 2009). Thus, because of these properties, microemulsion systems remove organic material with minimum mechanical energy and even bring about improvements in reservoir wettability (Tanthakit et al. 2008; Quintero et al. 2011, 2013, 2015; Brege et al. 2015; Wang et al. 2016).
The use of microemulsion to remove oil-based filter cake was studied by Carrasquilla et al. (2012), removing 100% of the filter cake in five minutes and presenting a water-wettable surface with a contact angle of 54.5°. Quintero et al. (2015) reported that a microemulsion flushing fluid has low surface tension and high solubility, promoting the removal of the oil-based filter cake, while the wettability tests indicated that the contact angle value below 10° is an important factor for obtaining well-cementing operations.
Curbelo et al. (2018) have developed two ternary diagrams using the surfactant Tween 80 and Ultranex NP 150, using a glycerin solution as aqueous phase and pine oil as oil phase. Two reverse micelle formulations were chosen by the authors and both removed 100% of the filter cake in 207 s for the Tween 80 system and 120 s for the NP 150 system. In the works developed by Wanderley Neto et al. (2020), the authors used microemulsified systems with dodecylammonium chloride (DAC), a cationic surfactant, and obtained 100% efficiency of filter cake removal using an oil-in-water system.
Previous research has investigated microemulsified systems with nonionic and cationic surfactants, so this work aimed to use an anionic surfactant which, in addition to being the cheapest possible, is also widely used in the industry; hence, sodium dodecyl sulfate was chosen as the anionic surfactant. Sodium dodecyl sulfate (SDS) is one of the cheapest surfactants available and is well known when it comes to its properties (Rühling et al. 2015). This surfactant is widely used in detergents, shampoos, toothpaste and various cosmetics, showing low toxicity (LD50 = 1288 mg/kg—orally in rats) (Walker et al. 1967; Singer and Tjeerdema 1993; Arslan et al. 2018).
Therefore, based on this context the present work aimed to develop microemulsion systems composed of direct, bicontinuous and reverse micelles from pseudoternary diagrams using biodegradable anionic surfactant sodium dodecyl sulfate (Singer and Tjeerdema 1993), n-butanol as cosurfactant, water as the aqueous phase and kerosene as the oil phase. The microemulsions were used as a flushing fluid to remove oil-based filter cake, and its compatibility with the cement slurry and its ability to increase the wettability of the rocks to be water wettable were evaluated, as important factors for well cementation.
Materials and methods
Materials
The reagents used to formulate the microemulsified systems were sodium dodecyl sulfate (SDS) with 90% purity (Dinâmica, Brazil), commercial kerosene (Líder, Brazil), n-butanol PA (99.4% purity) (Vetec, Brazil) and distilled water. Portland cement special class (like class A) was used to formulate the cement slurries. The non-aqueous drilling fluid used in the efficiency tests is a typical drilling fluid used in the state of Rio Grande do Norte, Brazil, and was supplied by Petrobras SA.
Methods
Microemulsion systems
Determination of the microemulsion region
From the pseudoternary diagram, the microemulsions were determined and obtained based on proportions of composition within its constituents. The overall solubility of the n-butanol/sodium dodecyl sulfate (C/S) combination in distilled water (AP) and kerosene (OP) was determined by mass titration. Then, distinct combinations of C/S and OP were weighed and titrated by the AP. Therefore, the boundary between Winsor regions (Winsor 1948) was determined by identifying phase separations of the mixture (De Castro Dantas et al. 2001; Thampi et al. 2014). Analytical balance (Precisa, Brazil, model 240 A), tube shaker (Fisatom) and centrifuge (Dayki) were used for the execution of the experiment.
Surface tension analysis
Microemulsions were characterized by their surface tensions, determined with the method of maximum pressure of the bubble by the flow of nitrogen gas, using a SensaDyne QC-6000 tensiometer, from Chem-Dyne Research Corp., aided by Software Features version 1.21. The measurements were performed at 25 °C ± 1ºC, and the results were obtained in mN/m (Roberto et al. 2013).
Droplet size
The microemulsions micelle sizes and distributions (polydispersion index) were determined with a dynamic light scattering technique, through the Zeta Potential Analyzer, model Zeta Plus 90 Bi-But. The measurements were performed at 5 scans of 30 s, angle of 15°, the temperature of 30 °C, laser of 40 mW, wavelength of 640 nm and accuracy of ± 3%.
Thermal stability
The microemulsion thermal stabilities were analyzed and determined using the thermogravimetry technique. The analyses were carried out by monitoring the mass loss as a function of time and/or temperature through the thermogravimetric analyzer, from TA Instruments® model SDTQ 600. With the aid of a thermobalance, a mass of 5 mg of microemulsion was weighted and used, at a heating rate of 10 °C/min in nitrogen atmosphere, a flow rate of 100 mL/min, until reaching the temperature of 600 °C.
Efficiency tests of filter cake removal
The efficiency of filter cake removal/washing by the application of microemulsions was evaluated following the procedure from test M12, PROCELAB (Laboratory Procedures and Methods Applied to Cementing Oil Wells), titled "Determination of removal efficiency for washing liquids used in oil wells cementing" (Campos 2009).
Initially, the retort test was performed on the drilling fluid using the Retort Offite Kit to obtain the amount of liquids and solids phases in the fluid. The retort test is analogous to a distiller that, from a high temperature, evaporates the liquid phase of the drilling fluid and then condenses it, so that it is possible to obtain the percentages of the liquid and solid phases in the fluid of interest.
After the drilling fluid homogenization in a Hamilton-Beach agitator for 15 min, a 4 mL aliquot was taken to form the filter cake on the wall of a beaker with viewing windows, consisting of 66 squares of 1 cm2 each (Wanderley Neto et al. 2020). Then, about 200 mL of microemulsion was added to the beaker, and with the aid of a viscometer, Fann 35, the analysis was performed at 300 RPM for 10 min, the number of clean squares measured at 2, 5, 7 and 10 min, respectively. At the end of the test, the total number of clean squares in the window was counted and the percentage of clean area in relation to the total area was calculated, obtaining the removal efficiency value. Equation 1 shows the mathematical calculations (Campos 2009):
After the efficiency test, the formulations are centrifuged (2500 RPM for 10 min) to promote the separation of solids, from the filter cake, and the liquid phase, composed by the flushing fluid. Subsequently, it was verified that there was breakage of the system in the flushing fluid, i.e., phase separation. This parameter is important because the stable systems may be reused after the washing tests. Then, an aliquot of the flushing fluid was subjected to surface tension measurements.
Wettability
This analysis was carried out to identify the effect that the flushing fluid has on the formation in terms of the formation's wettability. The evaluation of wettability on the rock was determined using the sessile drop method, which consists of dripping the liquid on a solid surface, from which the contact angle is being determined through a camera coupled Krüss® Drop Shape Analysis equipment, model DAS 100. Therefore, in order to perform this test, the limestone rock was milled and pressed into pellets (13 mm in diameter and 3 mm thick), saturated with the microemulsified fluid and exposed then to the environment conditions for 24 h at 25 °C ± 1 ºC to dry. Then, the equipment drips a drop of water onto the pellet to determine the contact angle as described by Dantas et al. (2014).
Rheology and fluid compatibility
The rheological behaviors of the flushing fluid and cement slurry were determined according to the relationship between shear stress and shear rate. From the viscosity results, the behavior of each fluid and its mixtures were evaluated by the degree of compatibility. The mixtures of the fluids were obtained according to the API standards, in which it was suggested to homogenize and mix the cement slurry and flushing fluid in proportions (v/v) of 95/5, 75/25, 50/50, 25/75 and 5/95, respectively, at a temperature of 88 °C and inside an atmospheric consistometer during 1200 s (API 2013). Afterward, the mixtures were placed into a viscometer Fann 35 and the shear rates used were 3, 6, 30, 60, 100, 200 and 300 RPM with ascending and descending readings at a 10-s interval (Campos 2009). The cement slurry was obtained by mixing cement and water until the density reaches 1.87 g/cm3.
The cement slurry was prepared from weighing 355 g of water and 772 g of cement. A Chandler Model 3060 mixer was used for mixing where the cement was uniformly added to the water. The slurry was mixed at a speed of 4000 RPM in 15 s. Then, the mixer speed was changed to 12,000 RPM and the slurry was stirred for 35 s for complete homogeneity.
The Ostwald–de Waele model or power law consists in analyzing the apparent viscosity variation with shear rates according to Eq. 2 (Malkin and Isayev 2011; Mezger 2014; Pal et al. 2019):
where: μa is the apparent viscosity (Pa.s), K is the consistency index (Pa.sn), γ is the shear rate in s−1, and n is the flow index (dimensionless).
Compressive strength
The study of the compressive strength of cement is necessary to evaluate the influences and impacts of the flushing fluid on characteristics of the cement and to compare it with the standard used in the industry. This is because in the cementation process, contamination of cement slurry occurs by the mixture flushing fluids and the fine particles remaining from the removal of the filter cake.
Therefore, in order to evaluate the characteristics of the cement contaminated with flushing fluid, compressive strength tests were performed using ultrasonic method with the aid of ultrasonic cement analyzer (UCA) equipment, model 4262 from Chandler Engineering. A Portland cement of class A, with a density of 1.87 g/cm3, commonly used in cementing operations in Brazilian oil wells NBR 9831 of 2006, was used for this experiment. The formulation of the cement slurry used is already presented in "Rheology and fluid compatibility" section.
To evaluate the influence of flushing fluid on cementation, the cement slurry was contaminated with 10% flushing fluid. The experiment was carried out at a constant pressure of 3000 psi and was divided into two heating ramps, being the first going from 25 ºC to 85 ºC for 116 min and a second one going from 85 ºC to 112.22 ºC for 124 min. After reaching 240 min, the experiment followed without alteration of its variables until completing 24 h, as suggested by the API standard in Sect. 13.5, RP 10B-2, of 2013.
Reuse of flushing fluid
The microemulsions that obtained 100% removal efficiency were submitted to other washes, in order to observe the possibility of reusing the microemulsion, following the same methodology as the first wash. Then, after the first wash to remove the filter cake, the microemulsion was centrifuged and reused until it was destabilized; at this moment it was no longer used for washing.
Results and discussion
Filter cake removal efficiency and emulsion formulation system characterizations
Figure 1 presents the pseudoternary diagrams obtained from the sodium dodecyl sulfate surfactant (SDS), n-butanol as cosurfactant, distilled water with aqueous phase (AP) and kerosene as oil phase (OP), varying the C/S ratio (from 1, 2 and 4).
As shown in Fig. 1, while studying the variation of C/S ratio, it was observed that it had no significant influence on the biphasic and Winsor IV + S microemulsion regions. At the same time, the constituents of the diagram contributed considerable to obtaining a larger microemulsion region, which in turn facilitates in choosing the micelle to be applied for filter cake removal.
The retort test showed that the fluid under study contains 21% solids, 30% water and 49% oil phase, which proves that the fluid is a water in oil emulsion (oil based). Originally, this fluid was formulated with 40% water and 60% oil, but after its use and storage, it lost some fractions of the phases involved.
As shown in Fig. 1, formulations of the direct micelle (1, 4 and 7), bicontinuous micelle (2, 5 and 8) and inverse micelle (3, 6 and 9) were chosen to be applied in the filter cake removal. The micelles presented the following compositions: direct (45% of AP, 40% of C/S and 15% OP; bicontinuous (30% AP, 40% C/S and 30% OP); and inverse (15% AP, 40% C/S and 45% OP). Table 1 presents the results of filter cake removal efficiencies for the formulations chosen and represented in Fig. 1.
As can be seen in Table 1, the formulations with inverse and bicontinuous micelle removed 100% of the filter cake, justified by the higher quantity of kerosene and n-butanol, which are both solvents with high capacity to solubilize nonpolar substances. For these micelles, the variation of the C/S ratio did not influence the filter cake removal since they all reached the value of 100%.
The direct micelles behaved differently than the others since only point 7 removed 100% of the filter cake. Looking at Table 1, the efficiency of filter cake removal increases when the C/S ratio is increased, reaching 68% for the C/S = 1, 86% for C/S = 2 and 100% for C/S = 4. The increase in efficiency when the C/S ratio was increased can be associated with a higher quantity of n-butanol, which ends up reaching a sufficient amount to solubilize all the nonpolar fractions, while the aqueous phase solubilizes the polar fractions.
The micelles types and compositions are important factors in the removal of filter cake by solubilization. The combination of water, kerosene, n-butanol and SDS formed a chemical system which presented polar and nonpolar characteristics, which in turn is essential to remove and ease the solubilization of the constituents of the filter cake, because it is also made up of polar and nonpolar fluids.
After verifying that the direct micelle with a C/S ratio = 4 removed 100% of the filter cake, six formulations of direct micelles were tested in an attempt to obtain chemical formulation systems that present a higher percentage of water (consequently lower percentage of the oil and cosurfactant/surfactant phase), which guarantees cost reduction. Figure 2 presents the diagram with these formulations: A, B, C, D, E and F, which were chosen to be applied in the filter cake removal.
Table 2 presents the formulations (fixed OP, varying C/S and AP) and their filter cake removal efficiency values.
As observed in Table 2, as the water percentage is increased (which also decreases the C/S ratio), the filter cake removal efficiency decreases. This behavior is associated with a smaller quantity of micelles in the flushing fluid, resulting in a decrease in the action of these formulations on the filter cake surface that reduces the efficiency in removing the constituents of the drilling fluid.
Table 3 presents the formulations (fixed C/S, varying AP and OP) and their filter cake removal efficiency values.
Analyzing Table 3, it is observed that as the percentage of the oil phase increases, the aqueous phase decreases, and the removal efficiency of the filter cake increases. These results show that a minimum quantity of kerosene is required for the total removal of the filter cake, clearly indicating that only the surfactant (even being in higher percentages) is not enough to solubilize the polar and nonpolar fractions of the filter cake. The oil phase present in the micelle is responsible for solubilizing the polar fractions of the filter cake, and, in order to solubilize all of it, the microemulsion must have a composition of at least 10% of kerosene. Thus, the complete filter cake removal depends on the composition within the microemulsion constituents.
As in previous works (Carrasquilla et al. 2012; Curbelo et al. 2018), water-in-oil-type emulsion formulation systems have a greater removal capacity, given their high percentage of oil phase. In comparing the performance of oil-in-water systems, as studied by Wanderley Neto et al. 2020, it is observed that systems with cationic surfactants do better than systems with anionic surfactants, as they have a greater quantity of water and less C/S ratio. But anionic surfactants are vastly cheaper than cationic surfactants, especially SDS.
Microemulsions characterization
Analyzing the formulations studied, those that obtained 100% efficiency in removing the filter cake were chosen, so the formulations chosen were: 2, 3, 5, 6, 7, 8, 9 and F.
The microemulsion systems were characterized according to their surface tension values, droplet size and polydispersion (Table 4). The values of surface tension and droplet size have a standard deviation of ± 0.1 mN/m and ± 1.0 nm, respectively.
Analyzing Table 4, it was observed that the surface tension values did not undergo any significant alteration in their values between each other before the removal of the filter cake, and it is possible to observe that there was no significant variation for the different types of micelles. Meanwhile, after the use of the formulations in the filter cake removal, there was a slight increase in the values of surface tension which was caused by the incorporation of polar and nonpolar fractions of the filter cake.
As for the droplet sizes, the values are within the characteristics of micellar structures (Fanun 2009). The change in micelle type did not promote a significant change in droplet size. This behavior has been already reported in the literature (Murashova et al. 2014). However, the polydispersion values above 0.3 suggest different sizes of micelles, which characterize these systems as heterogeneous (Nemen and Lemos-Senna 2011).
Microemulsions were evaluated for their thermal stability through the analysis of thermogravimetric curves and their derivatives. Figure 3 shows the thermogravimetric curves and their derivatives (TGA/DTG).
When analyzing Fig. 3, it was perceived that there were three thermogravimetric events: the first between 40 ºC and 76 ºC, the second between 77 ºC to 165 ºC and the third between 185 ºC and 260 ºC. The first event was probably associated with water (boiling point of 100 ºC) and loss of butanol (boiling point of 117 ºC), while the second event can be related to butanol and kerosene loss (boiling point above of 150 ºC) and the third event occurs as a result of phase change of the surfactant (melting point of 210 ºC).
As observed in Fig. 3b, the first event evidenced a higher mass loss for the direct micelle, followed by the inverse and bicontinuous. This behavior occurs due to higher water content in the direct micelle (lower boiling point), while the inverse micelle has more kerosene (higher boiling point for the liquids). The higher thermal stability of the bicontinuous micelle can be associated with the micellar packaging, which makes the liquid phases more adherent to the micelle. For the influence of C/S ratio, all micelles types suffered an increase in mass loss when the C/S ratio (C/S = 1, 2 and 4) was increased, since higher quantity of n-butanol results in higher solvent evaporation. When compared to the others, the F formulation suffered greater mass losses due to its higher water content (less kerosene and C/S).
After the first event, there was a mischaracterization of the formulations and, thus, the second event refers to the loss of butanol and kerosene. The third event was associated with the phase change of the surfactant (melting point), where the curves show that there was a greater loss for the systems with lower C/S, justified by the greater amount of surfactants.
Up to 50° C, a condition commonly found in oil wells (Curbelo et al. 2018), the results demonstrated that formulated microemulsion systems are thermally stable with mass retention between 75 and 90%, a condition in which microemulsions do not lose their constituents to the point of destabilization of the system, which makes its application feasible in this scenario.
Wettability study
The tests were carried out in order to evaluate the wettability condition by simulating rock (limestone) in the form of pellets saturated with flushing fluid, supposedly after removal of the filter cake by the flushing fluid.
The evaluation of rock wettability by water, after being treated with the flushing fluid, was necessary to verify whether there will be an efficient adhesion of the cement slurry to the formation, a condition for good cementation. Table 5 shows the contact angle values that were collected every 12 s until a total time of 60 s (1 min). The values are the averages of the measurements in duplicates for each formulation. The values presented a standard deviation of ± 10%.
As shown in Table 5, the contact angles at time zero presented values up to 41.1°, being well below 90°, which are good conditions for the rock surface to be wettable by water as required (Pal et al. 2019). Thus, it was possible to state that the rock wettability was inverted (for water) after using the flushing fluid.
By comparing the different micelle types and the C/S ratio variation in the formulations, no significant differences were observed between the contact angle values presented, indicating that the wettability of the rock surface is as a result of the consequence of adsorption of the surfactant on the surface of the rock, and not the influence of the fluids that constitute the microemulsion.
Over time, the wettability of the rock surface by water increases rapidly until reaching 24 s. After 24 s, the wettability of the surface reached a value and remains constant. This behavior suggests that cement adhesion to the rock surface will be quick and possibly efficient.
Figure 4 shows the contact angle behavior with time variation of saturated limestone with SDS-formulated flushing fluids.
The wettability results of saturated limestone, with all formulated flushing fluids systems, presented a higher degree of water wettability which is an important and necessary parameter for better cement-formation adhesion.
Characterization of optimal flushing fluid
Analyzing the results presented, it is observed that formulation F was the one that obtained 100% efficiency in removing filter cake with a greater amount of aqueous phase. From this, the optimal formulation (F) was subjected to rheological and compatibility studies with the cement slurry, analysis of the compressive strength and reuse capacity of the microemulsified system in multiple washes.
Rheological study and fluids compatibility
The rheological behaviors of fluids when simulating a cementation operation were performed according to the API standards which is based on the study of each fluid behavior and its mixtures, adhering to the volumetric proportions (v/v): 95/5, 75/25, 50/50, 25/75 and 5/95, of cement slurry and washing fluid, respectively. Figure 5 illustrates the study of the rheological behavior of the fluids.
As shown in Fig. 5, the flow curve profiles suggest that cement slurry (CS) and mixtures CS/F (95/5) and CS/F (75/25) behave like non-Newtonian fluids, considering that the shear stress versus shear rate is not constant, while the flushing fluid (point F) and the mixtures CS/F (50/50), CS/F (25/75) and CS/F (5/95) behave like Newtonian fluids, whereby the shear stress versus shear rate is practically constant. Figure 6 presents the study of the rheological compatibility of the fluids.
As shown in Fig. 6, the viscosity curves of fluid mixtures have similar profiles (viscosity decreases with an increase in shear rate) for the cement slurry and flushing fluid, indicating that there was no phase separation, excessive viscosity, gelification, flocculation, sedimentation or hardening in the mixture, consequently, suggesting a complete fluid compatibility.
As noted in Fig. 6, the cement slurry curve was the upper limit, while the flushing fluid was the lower limit. The fluid mixtures were between the upper and lower limits, obeying the criteria that evaluate good rheological compatibility between the fluids according to Campos (2009).
The flow curves suggest that the microemulsion system, cement slurry and mixtures were pseudoplastic fluids since they all presented a sharp decrease in viscosity when the shear rate was increased. Initially, the solid particles were at rest and offer high resistance to the flow (high viscosity), being that this resistance diminished when the dispersed particles were linear or laminar, facilitating the gliding of particles and reducing its viscosity, as suggested by Pal et al. (2019).
The Ostwald–de Waele model or power law was used to determine the rheological parameters of the fluids: consistency index (K) and behavioral or flow index (n). Table 6 presents the results of these parameters.
Analyzing Table 6, it can be seen that in the point F flushing fluid, the flow index (n) is close to 1 (one) and the consistency index (K) is low, which provides lower flow resistance suggesting Newtonian behavior by the fluid. This behavior is important to increase mobility and faster spreading on the surface of the filter cake, increasing removal efficiency.
For the cement slurry, the flow rate (n) is low, while the fluid deviation from Newtonian behavior is high, an also having high consistency index (K), which contributes to increasing the flow resistance. These cement slurry parameters suggest non-Newtonian behavior and pseudoplastic nature which occurs when n assumes values less than one and greater than zero (0 ˂ n ˂ 1).
The rheological evaluation of the mixtures between the cement slurry (CS) and the point F flushing fluid was studied to understand the variation of viscosity (µ) as a function of shear rate (γ).
Compressive strength
The evaluation of the compressive strength of cement slurry and cement slurry contaminated with the formulation flushing fluid F was determined by ultrasonic method—ultrasonic cement analyzer (UCA). Figure 7 illustrates morphology images of the specimens of cement slurry and cement slurry contaminated with the flushing fluid after 24 h of curing.
In Fig. 7, it can observed that the image presents a dense structure without formation of micro-cavities, suggesting compatibility between the fluids, which indicates good performance of the cement in the well even with the contamination of the flushing fluid, hence providing a suitable condition for oil well cementation operations.
Figure 8 shows the compressive strength of cement slurry uncontaminated and contaminated with flushing fluid, through the study of compressibility as a function of time.
The result shows that the compressive strength of the cement slurry and a mixture of cement slurry and flushing fluid presented values of 18.64 MPa and 10.44 MPa, respectively. The value of 10.44 MPa is considered acceptable, since the minimum value of compressive strength for conventional cement established by the petroleum industry is 10.3 MPa, as described by Curbelo et al. (2018).
This reduction in compressive strength is probably due to the organic compounds present in microemulsion F (OP = 10% and cosurfactant = 24%) that when mixed with cement causes a delay in hydration reactions, as proposed by Soares et al. (2017).
Reuse of flushing fluids
In order to evaluate the reuse power of microemulsion for the filter cake removal, the F formulation (60% AP, 30% C/S and 10% OP) was reused in multiple washes until destabilization, highlighted by the phase separation. The F formulation was used five times, the first four being 100% efficient, while in the fifth reuse the microemulsion separated into two phases. The complete removal of the filter cake was obtained after 3 min at a rotation of 300 RPM.
The microemulsion was destabilized in the fifth wash; in this situation, many polar and nonpolar fractions have already been incorporated into the micelle, promoting the rupture of the bonds between the surfactant with the aqueous and oily phases.
Conclusions
The results of filter cake removal efficiency showed that the use of SDS as a surfactant reached 100% for systems with inverse and bicontinuous micelles, in any of the studied C/S ratios (1, 2 and 4). The systems with direct micelles, of greatest interest for the study, only reached this percentage of removal when using a C/S ratio equal to 4, so the cosurfactant has a lot of influence on the removal efficiency for the studied systems.
The F system (AP = 60%, C/S = 30% and OP = 10%) was used in the interaction tests with the cement paste. The results showed rheologically compatible with cement. The cement had acceptable compressibility values, showing thermal stability up to 50 ºC and left the rock surface water wettable, which are suitable conditions that make the formulation technically feasible.
Therefore, the study showed that microemulsion systems constituted by anionic surfactant (SDS) can be used as a new alternative of microemulsified systems for removing filter cake, emphasizing the importance of the C/S ratio for its use as an oil-in-water system, provided that there is enough butanol in the system.
Abbreviations
- DAC:
-
Dodecylammonium chloride
- SDS:
-
Sodium dodecyl sulfate
- LD50 :
-
Lethal dose to cause the death of 50% of a group of test animals
- C/S:
-
Cosurfactant/surfactant
- AP:
-
Aqueous phase
- OP:
-
Oil phase
- RPM:
-
Rotation per minute
- v/v:
-
Volume/volume
- API:
-
American Petroleum Institute
- UCA:
-
Ultrasonic cement analyzer
- TGA:
-
Thermogravimetric analysis
- DTG:
-
Differential thermal analysis
References
API (2013) R. P. 10B-2: Recommended Practice for Testing Well Cements. API Recomm Pract B 10:20
Arslan A, Topkaya E, Bingöl D, Veli S (2018) Removal of anionic surfactant sodium dodecyl sulfate from aqueous solutions by O3/UV/H2O2 advanced oxidation process: process optimization with response surface methodology approach. Sustain Environ Res 28:65–71. https://doi.org/10.1016/j.serj.2017.11.002
Aughenbaugh K, Nair SD, Cowan K, van Oort E (2014) Contamination of deepwater well cementations by synthetic-based drilling fluids. In: SPE deepwater drilling and completions conference. Society of Petroleum Engineers
Bageri BS, Mahmoud M, Abdulraheem A et al (2017) Single stage filter cake removal of barite weighted water based drilling fluid. J Pet Sci Eng 149:476–484. https://doi.org/10.1016/J.PETROL.2016.10.059
Brege JJ, Pietrangeli G, McKellar AJ, et al (2015) Fluid formulations for cleaning oil-based or synthetic oil-based mud filter cakes. 6
Campos G (2009) Procelab—Procedimentos e métodos de laboratório destinados à cimentação de poços de petróleo—M12. Rio de Janeiro
Carrasquilla J, Guillot DJ, Ali SA, Nguyen C (2012) Microemulsion technology for synthetic-based mud removal in well cementing operations. In: SPE deepwater drilling and completions conference. Society of Petroleum Engineers
Curbelo FDS, Garnica AIC, Araújo EA et al (2018) Vegetable oil-based preflush fluid in well cementing. J Pet Sci Eng 170:392–399. https://doi.org/10.1016/J.PETROL.2018.06.061
DantasDeda SilvaDantas Neto TNCDNNAA et al (2019) Treatment of drill cuttings using microemulsion. J Pet Explor Prod Technol. https://doi.org/10.1007/s13202-019-00813-3
DantasDe TNC, Dantas NetoMoura AAEF (2001) Microemulsion systems applied to breakdown petroleum emulsions. J Pet Sci Eng 32:145–149. https://doi.org/10.1016/S0920-4105(01)00156-5
DantasDe TNC, Soaresde OliveiraNeto APJAW et al (2014) Implementing new microemulsion systems in wettability inversion and oil recovery from carbonate reservoirs. Energy Fuels 28:6749–6759. https://doi.org/10.1021/ef501697x
De Castro DantasNeto TNAAD, De A, Moura MCP (2001) Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res 35:2219–2224. https://doi.org/10.1016/S0043-1354(00)00507-8
Fanun M (2009) Microemulsions: properties and applications, 1st edn. CRC Press, Boca Raton
Li M, Ou H, Li Z et al (2015) Contamination of cement slurries with diesel-based drilling fluids in a shale gas well. J Nat Gas Sci Eng 27:1312–1320. https://doi.org/10.1016/J.JNGSE.2015.08.010
Malkin AY, Isayev AI (2011) Rheology: concepts, methods, and applications, 3rd edn. Chemtec
Mezger TG (2014) The rheology handbook—for users of rotational and oscillatory rheometers, 4th edn. Vincentz Network, Hanover
Murashova NM, Levchishin SY, Yurtov EV (2014) Effect of bis-(2-ethylhexyl)phosphoric acid on sodium bis-(2-ethylhexyl)phosphate microemulsion for selective extraction of non-ferrous metals. J Surf Deterg 17:1249–1258. https://doi.org/10.1007/s11743-014-1598-x
Nemen D, Lemos-Senna E (2011) Preparação e caracterização de suspensões coloidais de nanocarreadores lipídicos contendo resveratrol destinados à administração cutânea. Quim Nova 34:408–413. https://doi.org/10.1590/S0100-40422011000300008
Pal N, Kumar S, Bera A, Mandal A (2019) Phase behaviour and characterization of microemulsion stabilized by a novel synthesized surfactant: Implications for enhanced oil recovery. Fuel 235:995–1009. https://doi.org/10.1016/J.FUEL.2018.08.100
Quintero L, Jones TA, Clark D, Twynam AJ (2007) NAF filter cake removal using microemulsion technology. In: European formation damage conference. Society of Petroleum Engineers
Quintero L, Jones TA, Pietrangeli G (2011) Phase boundaries of microemulsion systems help to increase productivity. In: SPE European formation damage conference. society of petroleum engineers
Quintero L, Passanha WD, Aubry E, Poitrenaud H (2015) Advanced microemulsion cleaner fluid applications in deepwater wells. In: OTC Brasil. Offshore technology conference
Quintero L, Pietrangeli G, Salager JL, Forgiarini A (2013) Optimization of microemulsion formulations with linker molecules. In: SPE European formation damage conference & exhibition. Society of Petroleum Engineers
Roberto EC, Wanderley Neto AO, Martínez-Huitle CA et al (2013) The effect of type of self-assembled system and pH on the efficiency of corrosion inhibition of carbon-steel surfaces. Prog Org Coat 76:1308–1315. https://doi.org/10.1016/J.PORGCOAT.2013.04.002
Rühling A, Galla H-J, Glorius F (2015) A remarkably simple hybrid surfactant-NHC ligand, its gold-complex, and application in micellar catalysis. Chem A Eur J 21:12291–12294. https://doi.org/10.1002/chem.201502542
Schramm LL (2000) Surfactants : fundamentals and applications in the petroleum industry. Cambridge University Press, Cambridge
Singer MM, Tjeerdema RS (1993) Fate and effects of the surfactant sodium dodecyl sulfate. Rev Environ Contam Toxicol 133:95–149
Soares AA, FreitasDe JCO, Melo DM, De A et al (2017) Cement slurry contamination with oil-based drilling fluids. J Pet Sci Eng 158:433–440. https://doi.org/10.1016/J.PETROL.2017.08.064
Tanthakit P, Chavadej S, Scamehorn JF et al (2008) Microemulsion formation and detergency with oily soil: IV. Effect of rinse cycle design. J Surf Deterg 11:117–128. https://doi.org/10.1007/s11743-008-1062-x
Thampi NV, Ojha K, Nair UG (2014) Effect of branched alcohols on phase behavior and physicochemical properties of Winsor IV microemulsions. J Surf Deterg 17:371–381. https://doi.org/10.1007/s11743-013-1482-0
Walker AIT, Brown VKH, Ferrigan LW et al (1967) Toxicity of sodium lauryl sulphate, sodium lauryl ethoxysulphate and corresponding surfactants derived from synthetic alcohols. Food Cosmet Toxicol 5:763–769. https://doi.org/10.1016/S0015-6264(67)83275-9
Wanderley Neto AO, da Silva VL, Rodrigues DV et al (2020) A novel oil-in-water microemulsion as a cementation flushing fluid for removing non-aqueous filter cake. J Pet Sci Eng 184:106536. https://doi.org/10.1016/j.petrol.2019.106536
Wang C, Meng R, Xiao F, Wang R (2016) Use of nanoemulsion for effective removal of both oil-based drilling fluid and filter cake. J Nat Gas Sci Eng 36:328–338. https://doi.org/10.1016/J.JNGSE.2016.10.035
Winsor PA (1948) Hydrotropy, solubilisation and related emulsification processes. Trans Faraday Soc 44:376. https://doi.org/10.1039/tf9484400376
Acknowledgements
The authors of this paper would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 for financial support. The authors would also like to thank the Surfactants Technology Lab (LTT) and Technological Nucleus in Cementing of Oil Wells (NTCPP), both from the Federal University of Rio Grande do Norte (UFRN), for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Silva, V.L., Ribeiro, L.S., de Oliveira Freitas, J.C. et al. Application of SDS surfactant microemulsion for removal of filter cake of oil-based drilling fluid: influence of cosurfactant. J Petrol Explor Prod Technol 10, 2845–2856 (2020). https://doi.org/10.1007/s13202-020-00952-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-00952-y