Abstract
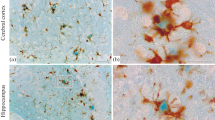
Although there are multiple histochemical tracers available to label plaques and tangles in the brain to evaluate neuropathology in Alzheimer disease (AD), few of them are versatile in nature and compatible with immunohistochemical procedures. Congo Red (CR) is an anisotropic organic stain discovered to label amyloid beta (Aβ) plaques in the brain. Unfortunately, its use is underappreciated due to its low resolution and brightness as stated in previous studies using bright field microscopy. Here, we modified a previous method to localize both plaques and tangles in brains from humans and a transgenic rodent model of AD for fluorescence microscopic visualization. The plaque staining affinities displayed by CR were compared with fibrillar pattern labeling seen with Thioflavin S. This study summarizes the optimization of protocols in which various parameters have been finetuned. To determine the target CR potentially binds, we have performed double labeling with different antibodies against Aβ as well as phosphorylated Tau. The plaque staining affinities exhibited by CR are compared with those associated with the diffuse pattern of labeling seen with antibodies directed against different epitopes of Aβ. Neither CP13, TNT2 or TOC1 binds all the neurofibrillary tangles as revealed by CR labeling in the human brain. Additionally, we also evaluated double labeling with AT8, AT180, and PHF1. Interestingly, PHF-1 shows 40% colocalization and AT8 shows 15% colocalization with NFT. Thus, CR is a much better marker to detect AD pathologies in human and rodent brains with higher fluorescence intensity relative to other conventional fluorescence markers.






Similar content being viewed by others
References
Bennhold H (1922) Specific staining of amyloid by Congo red. MuEnchener Medizinische Wochenschrift 69:1537–1538
Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T (2013) A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci 33:6245–6256
Combs B, Hamel C, Kanaan NM (2016) Pathological conformations involving the amino terminus of tau occur early in Alzheimer's disease and are differentially detected by monoclonal antibodies. Neurobiol Dis 94:18–31
Davies P (2000) Characterization and use of monoclonal antibodies to tau and paired helical filament tau. Methods Mol Med 32:361–373
DeLellis RA, Glenner GG, Ram JS (1968) Histochemical observations on amyloid with reference to polarization microscopy. J Histochem Cytochem 16:663–665
Divry P (1927) Etude histochimique des plaques seniles. J. Belge Neurol. Psychiatr 27:643–657
Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR (2003) Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 42:2768–2773
Glenner GG, Eanes ED, Page DL (1972) The relation of the properties of Congo red-stained amyloid fibrils to the -conformation. J Histochem Cytochem 20:821–826
Hatami A, Albay R 3rd, Monjazeb S, Milton S, Glabe C (2014) Monoclonal antibodies against Abeta42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain. J Biol Chem 289(32):131–143
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13:45–53
Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI (2011) Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci 31:9858–9868
Kelenyi G (1967) Thioflavin S fluorescent and Congo red anisotropic stainings in the histologic demonstration of amyloid. Acta Neuropathol 7:336–348
Klunk WE, Pettegrew JW, Abraham DJ (1989) Quantitative evaluation of congo red binding to amyloid-like proteins with a beta-pleated sheet conformation. J Histochem Cytochem 37:1273–1281
Liu L, Komatsu H, Murray IV, Axelsen PH (2008) Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J Mol Biol 377:1236–1250
Piekarska B, Rybarska J, Stopa B, Zemanek G, Krol M, Roterman I, Konieczny L (1999) Supramolecularity creates nonstandard protein ligands. Acta Biochim Pol 46:841–851
Puchtler H, Sweat F (1965) Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem 13:693–694
Roterman I, KrUl M, Nowak M, Konieczny L, Rybarska J, Stopa B, Piekarska B, Zemanek G (2001) Why Congo red binding is specific for amyloid proteins - model studies and a computer analysis approach. Med Sci Monit 7:771–784
Skowronek M, Roterman, Konieczny L, Stopa B, Rybarska J, Piekarska B, Gorecki A, Krol M (2000) The conformational characteristics of Congo red, Evans blue and Trypan blue. Comput Chem 24:429–450
Steensma DP (2001) “Congo” red: out of Africa? Arch Pathol Lab Med 125:250–252
Stopa B, Gorny M, Konieczny L, Piekarska B, Rybarska J, Skowronek M, Roterman I (1998) Supramolecular ligands: monomer structure and protein ligation capability. Biochimie 80:963–968
Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H (2002) Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol 61:282–293
Ward SM, Himmelstein DS, Lancia JK, Fu Y, Patterson KR, Binder LI (2013) TOC1: characterization of a selective oligomeric tau antibody. J Alzheimers Dis 37:593–602
Yakupova, E.I., Bobyleva, L.G., Vikhlyantsev, I.M., Bobylev, A.G., 2019. Congo Red and amyloids: history and relationship. Biosci Rep. 39.
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Author information
Authors and Affiliations
Contributions
Sumit Sarkar is the lead and corresponding author, primary input on experiment design, performed animal sacrifice, tissue processing and immune-labeling of histological sections, data collection and interpretation of photomicrographs and editing of manuscript. James Raymick-has significant input on histological labeling of sections, data collection. Elvis Cuevas has significant input involved in additional data collection and interpretation of photomicrographs and editing of manuscript. Hector Rosas-Hernandez has some input on writing, data collection and interpretation of photomicrographs and editing of manuscript. Joseph P. Hanig has significant input on experiment design, significant input as an author, interpretation experimental results and editing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors in this manuscript has any conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, S., Raymick, J., Cuevas, E. et al. Modification of methods to use Congo-red stain to simultaneously visualize amyloid plaques and tangles in human and rodent brain tissue sections. Metab Brain Dis 35, 1371–1383 (2020). https://doi.org/10.1007/s11011-020-00608-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-020-00608-0