Abstract
Purpose
To examine healing adaptations over 17 weeks post Achilles tendon (AT) rupture in the injured region (IR) compared to an uninjured region (UIR) of the AT.
Methods
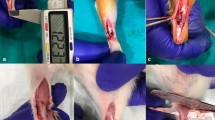
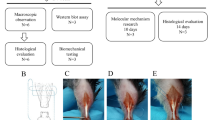
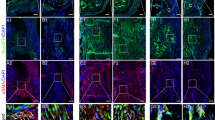
Twenty-four rats were subjected to a complete right-sided AT rupture, while the left side served as a control. ATs were harvested at 1, 2, 8 and 17 weeks post-rupture and stained with antibodies specific to Collagen type I (Col I) and II (Col II) as well as Alcian Blue and Picrosirius Red staining techniques. Histopathological changes, proteoglycan content, collagen alignment and immunoexpression were assessed.
Results
Both regions examined, IR and UIR, exhibited over weeks 1–17 similar healing adaptations of increasing collagen alignment, decreasing Col I immunoexpression, as well as increasing proteoglycan content and Col II occurrence. Increased proteoglycan content was found already at week 2 in the UIR, while it first increased at week 8 in the IR. The area positive to Col II was increased compared to controls at week 8 in the UIR, whereas it first raised at week 17 in the IR. Collagen disorganization successively declined to reach control levels at week 17 in the UIR, but was still higher in the IR.
Conclusion
This study demonstrated that uninjured areas of the AT remote from the rupture site also undergo pronounced remodeling, although with time-span differences relative to injured AT portions. These changes including the pathologic heterotopic mineralization and chondrogenic differentiation observed in both regions may have implications in the choice of rehabilitation regimes in order to prevent secondary rupture.







Similar content being viewed by others
References
Abraham T, Fong G, Scott A (2011) Second harmonic generation analysis of early Achilles tendinosis in response to in vivo mechanical loading. BMC Musculoskelet Disord 12:26
Aikawa E, Whittaker P, Farber M et al (2006) Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113:1344–1352
Asai S, Otsuru S, Candela ME, Enomoto-Iwamoto M et al (2014) Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells 32:3266–3277
Ateschrang A, Gratzer C, Weise K (2008) Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch Orthop Trauma Surg 128:1087–1092
Bring DK, Paulson K, Renstrom P et al (2012) Residual substance P levels after capsaicin treatment correlate with tendon repair. Wound Repair Regen 20:50–60
Burssens A, Forsyth R, Bongaerts W et al (2013) Arguments for an increasing differentiation towards fibrocartilaginous components in midportion Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc 21:1459–1467
Checa S, Rausch MK, Petersen A et al (2015) The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech Model Mechanobiol 14:1–13
Corradino B, Di Lorenzo S, Calamia C et al (2015) Surgical Repair Of Acute Achilles tendon rupture with an end-to-end tendon suture and tendon flap. Injury 46:1637–1640
de Mos M, Koevoet W, van Schie HT et al (2009) In vitro model to study chondrogenic differentiation in tendinopathy. Am J Sports Med 37:1214–1222
Dyment NA, Kazemi N, Aschbacher-Smith LE et al (2012) The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. J Orthop Res 30:28–36
Erdogan F, Aydingoz O, Kesmezacar H et al (2004) Calcification of the patellar tendon after ACL reconstruction. A case report with long-term follow-up. Knee Surg Sports Traumatol Arthrosc 12:277–279
Finlay S, Seedhom BB, Carey DO et al (2016) In vitro engineering of high modulus cartilage-like constructs. Tissue Eng Part C Methods 22:382–397
Hast MW, Zuskov A, Soslowsky LJ (2014) The role of animal models in tendon research. Bone Jt Res 3:193–202
Khayyeri H, Blomgran P, Hammerman M et al (2017) Achilles tendon compositional and structural properties are altered after unloading by botox. Sci Rep 7:13067
Lattouf R, Younes R, Lutomski D et al (2014) Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem 62:751–758
Lemme NJ, Li NY, DeFroda SF et al (2018) Epidemiology of Achilles tendon ruptures in the United States: athletic and nonathletic injuries from 2012 to 2016. Orthop J Sports Med 6:2325967118808238
Li HY, Hua YH (2016) Achilles tendinopathy: current concepts about the basic science and clinical treatments. Biomed Res Int 2016:6492597
Lin TW, Cardenas L, Soslowsky LJ (2004) Biomechanics of tendon injury and repair. J Biomech 37(6):865–877
Longo UG, Ronga M, Maffulli N (2009) Achilles tendinopathy. Sports Med Arthrosc Rev 17:112–126
Lui PP, Chan LS, Lee YW et al (2010) Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 49:231–239
Lui PP, Fu SC, Chan LS et al (2009) Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J Histochem Cytochem 57:91–100
Maffulli N (1999) Rupture of the Achilles tendon. J Bone Jt Surg Am 81:1019–1036
Magnusson SP, Langberg H, Kjaer M (2010) The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol 6:262–268
Milz S, Benjamin M, Putz R (2005) Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol 178:1–71
Müller SA, Todorov A, Heisterbach PE et al (2015) Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc 23:2097–2105
Olaleye OA, Zahn H (2008) An unusual second rupture of the Achilles tendon: a case report. Foot Ankle J 1:3
Runesson E, Ackermann P, Karlsson J et al (2015) Nucleostemin- and Oct 3/4-positive stem/progenitor cells exhibit disparate anatomical and temporal expression during rat Achilles tendon healing. BMC Musculoskelet Disord 16:212
Sagarriga Visconti C, Kavalkovich K, Wu J et al (1996) Biochemical analysis of collagens at the ligament-bone interface reveals presence of cartilage-specific collagens. Arch Biochem Biophys 328:135–142
Schriefl AJ, Reinisch AJ, Sankaran S et al (2012) Quantitative assessment of collagen fibre orientations from two-dimensional images of soft biological tissues. J R Soc Interface 9:3081–3093
Sharma P, Maffulli N (2006) Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 6:181–190
Silva FS, Bortolin RH, Araújo DN et al (2017) Exercise training ameliorates matrix metalloproteinases 2 and 9 messenger RNA expression and mitigates adverse left ventricular remodeling in streptozotocin-induced diabetic rats. Cardiovasc Pathol 29:37–44
Tekari A, Luginbuehl R, Hofstetter W et al (2014) Chondrocytes expressing intracellular collagen type II enter the cell cycle and co-express collagen type I in monolayer culture. J Orthop Res 32:1503–1511
Voleti PB, Buckley MR, Soslowsky LJ (2012) Tendon healing: repair and regeneration. Annu Rev Biomed Eng 14:47–71
Yang G, Rothrauff BB, Tuan RS (2013) Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today 99:203–222
Zhang C, Zhang Y, Zhong B et al (2016) SMAD7 prevents heterotopic ossification in a rat Achilles tendon injury model via regulation of endothelial-mesenchymal transition. FEBS J 283:1275–1285
Acknowledgements
The authors are grateful to Eva Runesson for her assistance in preparing this manuscript.
Funding
This Research had support of the Sahlgrenska University Hospital, Gothenburg, Sweden (ALF project 164031, ALFGBG-442011).
Author information
Authors and Affiliations
Contributions
BIE and PWA conceived and designed the study. FSS performed the data collection. FSS and BJA performed the data analysis and statistics, prepared figures, and wrote the first draft of this manuscript. BIE and PWA supervised the preparation of the manuscript and contributed to interpretation of the results. All authors approved the final manuscript and confirmed the responsibility of the content of this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests regarding the publication of this paper.
Ethical approval
The Gothenburg Ethical Committee on Animal Research approved this study (Dnr. 257-2009).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, F.S., Abreu, B.J., Eriksson, B.I. et al. Complete mid-portion rupture of the rat achilles tendon leads to remote and time-mismatched changes in uninjured regions. Knee Surg Sports Traumatol Arthrosc 29, 1990–1999 (2021). https://doi.org/10.1007/s00167-020-06239-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-06239-3