Abstract
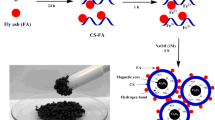
Crosslinked chitosan-ethylene glycol diglycidyl ether (CTS-EGDE) was modified by loading fly ash (FA) particles into its polymeric matrix to improve the adsorptive removal of reactive red 120 (RR120) dye. The Box–Behnken design was used to assist in the tuning of optimum synthesis and adsorption conditions, such as the loading ratio of FA particles (A: 0–50%), adsorbent dose (B: 0.02–0.08 g), solution pH (C: 4–10), temperature (D: 30 °C–60 °C), and time (E: 20–60 min). The highest removal rate (98.8%) of 50 mg/L RR120 dye was achieved under the following conditions: FA loading, 25% (CTS-EGDE/FA-25); adsorbent dose, 0.05 g; solution pH, 4; temperature, 60 °C; and time, 40 min. The maximum adsorption capacity of CTS-EGDE/FA-25 for RR120 was 220 mg/g at 60 °C. This work provides insights into the optimization of the synthesis of composite materials, which can potentially be applied in wastewater treatment.







Similar content being viewed by others
References
Roy U, Sengupta S, Banerjee P, Das P, Bhowal A, Datta S (2018) Assessment on the decolourization of textile dye (Reactive Yellow) using Pseudomonas sp. immobilized on fly ash: response surface methodology optimization and toxicity evaluation. J Environ Manage 223:185–195
Ranjbari S, Tanhaei B, Ayati A, Sillanpää M (2019) Novel Aliquat-336 impregnated chitosan beads for the adsorptive removal of anionic azo dyes. Int J Biol Macromol 125:989–998
de Figueiredo NT, Dalarme NB, da Silva PMM, Landers R, Picone CSF, Prediger P (2020) Novel magnetic chitosan/quaternary ammonium salt graphene oxide composite applied to dye removal. J Environ Chem Eng 8:103820
Unal BO, Bilici Z, Ugur N, Isik Z, Harputlu E, Dizge N, Ocakoglu K (2019) Adsorption and Fenton oxidation of azo dyes by magnetite nanoparticles deposited on a glass substrate. J Water Process Eng 32:100897
Kaur K, Badru R, Singh PP, Kaushal S (2020) Photodegradation of organic pollutants using heterojunctions: a review. J Environ Chem Eng 8:103666
Dotto J, Fagundes-Klen MR, Veit MT, Palácio SM, Bergamasco R (2019) Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J Clean Prod 208:656–665
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surface Interf 18:100422
Xu R, Wang J, Chen D, Liu T, Zheng Z, Yang F, Xiang M (2020) Preparation and performance of a charge-mosaic nanofiltration membrane with novel salt concentration sensitivity for the separation of salts and dyes. J Membr Sci 595:117472
Awad AM, Jalab R, Benamor A, Naser MS, Ba-Abbad MM, El-Naas M, Mohammad AW (2020) Adsorption of organic pollutants by nanomaterial-based adsorbents: an overview. J Mol Liq 301:112335
Rani M, Shanker U (2020) Metal oxide-chitosan based nanocomposites for efficient degradation of carcinogenic PAHs. J Environ Chem Eng 8:103810
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol 142:732–741
Abdulhameed AS, Jawad AH, Mohammad AT (2019) Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresour Technol 293:122071
Shi Y, Hu H, Ren H (2020) Dissolved organic matter (DOM) removal from biotreated coking wastewater by chitosan-modified biochar: adsorption fractions and mechanisms. Bioresour Technol 297:122281
Zheng C, Zheng H, Sun Y, Xu B, Wang Y, Zheng X, Wang Y (2019) Simultaneous adsorption and reduction of hexavalent chromium on the poly (4-vinyl pyridine) decorated magnetic chitosan biopolymer in aqueous solution. Bioresour Technol 293:122038
Jawad AH (2019) Adsorption and mechanism study for methyl orange dye by cross-linked chitosan-ethylene glycol diglycidyl ether beads. Desalin Water Treat 166:377–386
Yu S, Cui J, Wang J, Zhong C, Wang X, Wang N (2020) Facile fabrication of Cu (II) coordinated chitosan-based magnetic material for effective adsorption of reactive brilliant red from aqueous solution. Int J Biol Macromol 149:562–571
Mokhtar A, Abdelkrim S, Djelad A, Sardi A, Boukoussa B, Sassi M, Bengueddach A (2020) Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr Polym 229:115399
Nawi MA, Jawad AH, Sabar S, Ngah WSW (2011) Photocatalytic-oxidation of solid state chitosan by immobilized bilayer assembly of TiO2-chitosan under a compact household fluorescent lamp irradiation. Carbohydr Polym 83(3):1146–1152
Yildirim A, Bulut Y (2020) Adsorption behaviors of malachite green by using crosslinked chitosan/polyacrylic acid/bentonite composites with different ratios. Environ Technol Innov 17:100560
Tahira I, Aslam Z, Abbas A, Monim-ul-Mehboob M, Ali S, Asghar A (2019) Adsorptive removal of acidic dye onto grafted chitosan: a plausible grafting and adsorption mechanism. Int J Biol Macromol 136:1209–1218
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Mohammad AT, Abdulhameed AS, Jawad AH (2019) Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: methyl orange adsorption and mechanism studies. Int J Biol Macromol 129:98–109
Chen G, Song K, Huang X, Wang W (2019) Removal of toluene and Pb (II) using a novel adsorbent modified by titanium dioxide and chitosan. J Mol Liq 295:111683
Jawad AH, Malek NNA, Abdulhameed AS, Razuan R (2020) Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of reactive orange 16 dye: optimization by Box-Behnken design. J Polym Environ 28(3):1068–1082
Marnani NN, Shahbazi A (2019) A novel environmental-friendly nanobiocomposite synthesis by EDTA and chitosan functionalized magnetic graphene oxide for high removal of Rhodamine B: adsorption mechanism and separation property. Chemosphere 218:715–725
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Mesoporous crosslinked chitosan-activated charcoal composite for the removal of thionine cationic dye: comprehensive adsorption and mechanism study. J Polym Environ 28(3):1095–1105
Jimtaisong A, Sarakonsri T (2019) Chitosan intercalated bentonite as natural adsorbent matrix for water-soluble sappanwood dye. Int J Biol Macromol 129:737–743
Zhao R, Ma T, Zhao S, Rong H, Tian Y, Zhu G (2020) Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem Eng J 382:122893
Moradian M, Hu Q, Aboustait M, Robertson B, Ley MT, Hanan JC, Xiao X (2019) Direct in-situ observation of early age void evolution in sustainable cement paste containing fly ash or limestone. Compos B 175:107099
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36(3):327–363
Adamczuk A, Kołodyńska D (2015) Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem Eng J 274:200–212
Agarwal S, Rajoria P, Rani A (2018) Adsorption of tannic acid from aqueous solution onto chitosan/NaOH/fly ash composites: equilibrium, kinetics, thermodynamics and modeling. J Environ Chem Eng 6(1):1486–1499
Mu C, Zhang L, Zhang X, Zhong L, Li Y (2020) Selective adsorption of Ag (I) from aqueous solutions using chitosan/polydopamine@ C@ magnetic fly ash adsorbent beads. J Hazard Mater 381:120943
Malek NNA, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH (2020) New magnetic Schiff's base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol 146:530–539
Kang X, Xia Z, Chen R, Liu P, Yang W (2019) Effects of inorganic cations and organic polymers on the physicochemical properties and microfabrics of kaolinite suspensions. Appl Clay Sci 176:38–48
Kang X, Xia Z, Chen R, Sun H, Yang W (2019) Effects of inorganic ions, organic polymers, and fly ashes on the sedimentation characteristics of kaolinite suspensions. Appl Clay Sci 181:105220
Mourak A, Hajjaji M, Alagui A (2019) Cured cuttlebone/chitosan-heated clay composites: microstructural characterization and practical performances. J Build Eng 26:100872
Kan LL, Lv JW, Duan BB, Wu M (2019) Self-healing of engineered geopolymer composites prepared by fly ash and metakaolin. Cem Concr Res 125:105895
Vieira RS, Beppu MM (2006) Interaction of natural and crosslinked chitosan membranes with Hg (II) ions. Colloids Surf A Physicochem Eng Asp 279(1–3):196–207
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Wen Y, Tang Z, Chen Y, Gu Y (2011) Adsorption of Cr (VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chem Eng J 175:110–116
Li Z, Chen R, Zhang L (2013) Utilization of chitosan biopolymer to enhance fly ash-based geopolymer. J Mater Sci 48(22):7986–7993
Singh K, Gupta AB, Sharma AK (2016) Fly ash as low cost adsorbent for treatment of effluent of handmade paper industry-kinetic and modelling studies for direct black dye. J Clean Prod 112:1227–1240
Pandey S, Tiwari S (2015) Facile approach to synthesize chitosan based composite—characterization and cadmium (II) ion adsorption studies. Carbohydr Polym 134:646–656
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J Clean Prod 232:43–56
Kumar M, Dosanjh HS, Singh H (2018) Magnetic zinc ferrite–alginic biopolymer composite: as an alternative adsorbent for the removal of dyes in single and ternary dye system. J Inorg Organomet Polym Mater 28(5):1688–1705
Kumar M, Dosanjh HS, Singh H (2018) Magnetic zinc ferrite–chitosan bio-composite: synthesis, characterization and adsorption behavior studies for cationic dyes in single and binary systems. J Inorg Organomet Polym Mater 28(3):880–898
Kumar M, Dosanjh HS, Singh H (2018) Removal of lead and copper metal ions in single and binary systems using biopolymer modified spinel ferrite. J Environ Chem Eng 6(5):6194–6206
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14(1):305–313
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ren F, Li Z, Tan WZ, Liu XH, Sun ZF, Ren PG, Yan DX (2018) Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J colloid Interf Sci 532:58–67
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Oyekanmi AA, Ahmad A, Hossain K, Rafatullah M (2019) Statistical optimization for adsorption of Rhodamine B dye from aqueous solutions. J Mol Liq 281:48–58
Çelekli A, Al-Nuaimi AI, Bozkurt H (2019) Adsorption kinetic and isotherms of reactive red 120 on moringa oleifera seed as an eco-friendly process. J Mol Struct 1195:168–178
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye reactive red 120 by the chitosan–Fe (III)-crosslinked: batch and fixed-bed studies. J Environ Chem Eng 1:1350–1358
Munagapati VS, Wen JC, Pan CL, Gutha Y, Wen JH (2019) Enhanced adsorption performance of reactive red 120 azo dye from aqueous solution using quaternary amine modified orange peel powder. J Mol Liq 285:375–385
Cardoso NF, Lima EC, Royer B, Bach MV, Dotto GL, Pinto LA, Calvete T (2012) Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of reactive red 120 dye from aqueous effluents. J Hazard Mater 241:146–153
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Hybrid crosslinked Chitosan epichlorohydrin/TiO2 nanocomposite for reactive red 120 dye adsorption: kinetic, isotherm, thermodynamic, and mechanism study. J Polym Environ 28:624–637
Prola LD, Acayanka E, Lima EC, Umpierres CS, Vaghetti JC, Santos WO, Djifon PT (2013) Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of reactive red 120 textile dye from aqueous solution. Ind Crops Prod 46:328–340
Pereira IC, Carvalho KQ, Passig FH, Ferreira RC, Rizzo-Domingues RCP, Hoppen MI, Perretto F (2018) Thermal and thermal-acid treated sewage sludge for the removal of dye reactive red 120: characteristics, kinetics, isotherms, thermodynamics and response surface methodology design. J Environ Chem Eng 6(6):7233–7246
Acknowledgements
The authors would like to thank Ministry of Education, Malaysia for supporting this research project under fundamental research Grant scheme (600-IRMI/FRGS/5/3 (340/2019); FRGS/1/2019/STG01/UiTM/02/3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jawad, A.H., Mohammed, I.A. & Abdulhameed, A.S. Tuning of Fly Ash Loading into Chitosan-Ethylene Glycol Diglycidyl Ether Composite for Enhanced Removal of Reactive Red 120 Dye: Optimization Using the Box–Behnken Design. J Polym Environ 28, 2720–2733 (2020). https://doi.org/10.1007/s10924-020-01804-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01804-w