Abstract
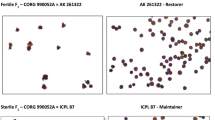
Baby corn has become a popular dietary choice worldwide. Here, we evaluated the potential of different male sterility systems as a tool to save cost for manual detasseling in baby corn. 24 hybrids having fertile (N-) and different sterile (T-, C- and S-) cytoplasms were analyzed for pollen sterility and various baby corn traits under three dates of sowing. ANOVA revealed significant genetic variation for number of baby corn ears, baby corn yield, length and width of baby corn ears, fodder weight and silking time. Cytoplasm and date of sowing caused significant variation for majority of baby corn characters. All the T-cytoplasm based hybrids showed no exertion of anthers and complete sterility except in one combination. C-cytoplasm based hybrids showed no to partial anther exertion and complete sterility to partial fertility of pollen. The S-type cytoplasm was quite unstable and showed partial to complete anther exertion and pollen fertility. Across hybrids and sowing time, T-cytoplasm had the highest number of baby corn ears per plot (61.83) and baby corn weight (2659.61 kg/ha) than C-cytoplasm (58.61, 2460.17 kg/ha), N-cytoplasm (57.39, 2323.08 kg/ha) and S-cytoplasm (56.83, 2301.89 kg/ha). The highest baby corn ear -length (8.82 cm), -diameter (1.48 cm), and fodder weight (35.07 t/ha) were recorded in T-cytoplasm. Late sowing had positive effects on baby corn over early planting. This information provides great significance for the utilization of male sterility in baby corn breeding. This is the first report on effects of different male sterility systems as a potential genetic emasculation mechanism in baby corn cultivation.


Similar content being viewed by others
Data availability
All supporting data are included within the article and its additional files.
References
Aekatasanawan C, Chowchong S, Jampatong S, Balla C (1994) Utilization of male sterility for baby corn improvement. Kasetsart J 28:167–170
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Bozinovic S, Prodanovic S, Vancetovic J, Nikolic A, Ristic D, Kostadinovic M, Ignjatovic D (2015) Individual and combined (Plus-hybrid) effect of cytoplasmic male sterility and xenia on maize grain yield. Chil J Agri Res 75:160–167. https://doi.org/10.4067/S0718-58392015000200004
Calugar RE, Has VV, Varga A, Vana CD, Copandean A, Has I (2018) The role of cytoplasmatic diversification on some productivity traits of maize. Euphytica 214:90. https://doi.org/10.1007/s10681-018-2171-x
Chinwuba PMGCO, Zuber MS (1961) Interaction of detasseling, sterility and spacing on yield of maize hybrids. Crop Sci 1:279–280. https://doi.org/10.2135/cropsci1961.0011183X000100040015x
Choudhary M, Kumar B, Kumar P, Guleria SK, Singh NK, Khulbe R, Kamboj MC, Vyas M, Srivastava RK, Puttaramanaik Swain D, Mahajan V, Rakshit S (2019) GGE biplot analysis of genotype × environment interaction and identification of mega-environment for baby corn hybrids evaluation in India. Indian J Genet 79:658–669
Dass S, Yadav VK, Kwatra A, Jat ML, Rakshit S, Kaul J, Parkash O, Singh I, Singh KP, Sekhar JC (2008) Baby corn in India. Technical Bulletin, Directorate of Maize Research, Pusa Campus, New Delhi 6:1–45
Dewey RE, Levings ICS, Timothy DH (1986) Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell 44:439–449
Dill CL, Wise RP, Schnable PS (1997) Rf8 and Rf* mediate unique T-urf13-transcript accumulation, revealing a conserved motif associated with RNA processing and restoration of pollen fertility in T-cytoplasm maize. Genetics 147:1367–1379
Duvick DN (1972) Potential usefulness of new cytoplasmic male sterile and sterility systems. In: Proceedings of 27th Ann Corn and Sorghum Res conference, vol 27, pp 197–201
Gabay-Laughnan S, Chase CD, Ortega VM, Zhao L (2004) Molecular-genetic characterization of CMS-S restorer-of-fertility alleles identified in Mexican maize and teosinte. Genetics 166:959–970. https://doi.org/10.1534/genetics.166.2.959
Gabay-Laughnan S, Laughnan JR (1994) Male sterility and restorer genes in maize. The maize handbook. Springer, New York, pp 418–423
Grogan CO (1956) Detasseling responses in corn. Agron J 48:247–249. https://doi.org/10.2134/agronj1956.00021962004800060001x
Hu YM, Tang JH, Yang H, Xie HL, Lu XM, Niu JH, Chen WC (2006) Identification and mapping of Rf-I an inhibitor of the Rf5 restorer gene for CMS-C in maize (Zea mays L.). Theor Appl Genet 113:357–360. https://doi.org/10.1007/s00122-006-0302-6
Iddumu V, Gogoi R, Hossain F, Aggarwal R, Kumar A, Mandal PK (2019) Confirmation of pathogenic race of Bipolarismaydis causing maydis leaf blight of maize in India. In: Abstracts: XIV agricultural science congress, 20--23 February, 2019, NASC, Pusa, New Delhi, India, p 704
Kaeser O, Weingartner U, Camp KH, Chowchong S (2003) Impact of different CMS types on grain yield of dent x flint hybrids of maize (Zea mays L.). Maydica 48:15–20
Levings CS (1990) The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science 250:942–947. https://doi.org/10.1126/science.250.4983.942
Moreira JN, Silva PSL, Silva K, Dombroski JL, Castro RS (2010) Effect of detasseling on baby corn, green ear and grain yield of two maize hybrids. Hortic Bras 28:406–411. https://doi.org/10.1590/S0102-05362010000400005
Prakash NR, Chhabra R, Zunjare RU, Muthusamy V, Hossain F (2020) Molecular characterization of teosinte branched1 gene governing branching architecture in cultivated maize and wild relatives. 3Biotech 10:77. https://doi.org/10.1007/s13205-020-2052-6
Prakash NR, Zunjare RU, Muthusamy V, Chand G, Kamboj MC, Bhat JS, Hossain F (2019) Genetic analysis of prolificacy in ‘Sikkim Primitive—a prolific maize (Zea mays L.) landrace of North-Eastern Himalaya. Plant Breed 138:781–789. https://doi.org/10.1111/pbr.12736
Raj KG, Virmani SS (1988) Genetic of fertility restoration of ‘WA’type cytoplasmic male sterility in rice. Crop Sci. 28:787–792. https://doi.org/10.2135/cropsci1988.0011183X002800050013x
Ramachandrappa BK, Nanjappa HV, Thimmegowda MN, Soumya TM (2004) Production management for profitable babycorn cultivation. Indian Farm 54:3–7
Rhoades MM (1933) The cytoplasmic inheritance of male sterility in Zea mays. J Genet 27:71–93
Rodrigues LA, Von Pinho RG, Junior LAYB, de Souza VF, Pereira FC, de Brito AH, Filho EMF (2018) Influence of cytoplasmic genetic male sterility in the grain yield of maize hybrids. Afr J Agric Res 13(45):2610–2617. https://doi.org/10.5897/AJAR2018.13381
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Stamp P, Chowchong S, Menzi M, Weingartner U, Kaeser O (2000) Increase in the yield of cytoplasmic male sterile maize revisited. Crop Sci 40:1586–1587. https://doi.org/10.2135/cropsci2000.4061586x
Stevanovic M, Camdzija Z, Pavlov J, Crevar M, Grcic N, Stankovic G, Mladenovic DS (2013) The effect of cytoplasmic male sterility on yield stability of maize inbred lines. In: IV International symposium “Agrosym 2013", October 3–6, Jahorina, Bosnia and Herzegovina. Conference book, pp 95–100
Stevanovic M, Camdzija Z, Pavlov J, Markovic K, Vancetovic J, Mladenovic D, Filipovic M (2016) The application of protein markers in conversion of maize inbred lines to the cytoplasmic male sterility basis. Genetika 48:691–698. https://doi.org/10.2298/GENSR1602691S
Su A, Song W, Xing J, Zhao Y, Zhang R, Li C, Duan M, Luo M, Shi Z, Zhao J (2016) Identification of genes potentially associated with the fertility instability of S-type cytoplasmic male sterility in maize via bulked segregant RNA-Seq. PLoS ONE 11(9):e0163489. https://doi.org/10.1371/journal.pone.0163489
Tracy WF, Everett HL, Gracen VE (1991) Inheritance, environmental effects, and partial male fertility in C-type CMS in a maize inbred. J Hered 82:343–346. https://doi.org/10.1093/oxfordjournals.jhered.a111096
Weider C, Stamp P, Christov N, Hüsken A, Foueillassar X, Camp KH, Munsch M (2009) Stability of cytoplasmic male sterility in maize under different environmental conditions. Crop Sci 49:77–84. https://doi.org/10.2135/cropsci2007.12.0694
Zabala G, Gabay-Laughnan S, Laughnan JR (1997) The nuclear gene Rf3 affects the expression of the mitochondrial chimeric sequence R implicated in S-type male sterility in maize. Genetics 147:847–860
Acknowledgements
The first author is thankful to Indian Council of Agricultural Research for providing financial assistance through Junior Research Fellowship during the M.Sc. programme. The support of ICAR-IIMR-Winter Nursery Centre, Hyderabad for providing off-season nursery is duly acknowledged. Thanks are due to Maize Genetics Cooperation Stock Center, Urbana, USA for providing the source germplasm for different male sterility systems. We also thank CCS-HAU, Uchani for providing the parental lines of HM4 and HM8 hybrids.
Funding
The financial support, and field and lab facilities provided by ICAR-Indian Agricultural Research Institute, New Delhi are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Conduct of experiments: SP; Development of hybrids: RUZ; Phenotypic data recording: HD and MGM; Pollen fertility study: SP and PKB; Raising of trials: RK, VB and VM; Statistical analyses: VM; Manuscript preparation: SP, VM and FH; Design of experiments: VM and FH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Consent for publication
All the authors have read the content and consented to submit the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pal, S., Zunjare, R.U., Muthusamy, V. et al. Influence of T-, C- and S- cytoplasms on male sterility and their utilisation in baby corn hybrid breeding. Euphytica 216, 146 (2020). https://doi.org/10.1007/s10681-020-02682-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02682-y