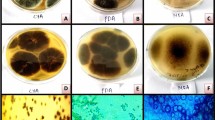
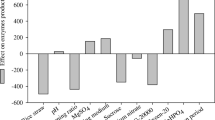
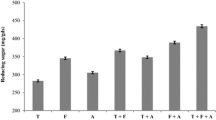
Abstract
The cellulase production by filamentous fungi Aspergillus fumigatus JCM 10253 was carried out using agro-industrial waste ragi husk as a substrate in the microbial fermentation. The effect of the process parameters such as temperature, substrate concentration, pH, and incubation process time and their interdependence was studied using response surface methodology. The optimum cellulase activities were obtained at 50 °C under the conditions with 1–2% of substrate concentration at pH 2–4 for the incubation period of 7–8 days. The maximum carboxymethyl cellulase (CMCase) and β-glucosidase activities with optimized process variables were 95.2 IU/mL and 0.174 IU/mL, respectively. The morphological characterization of fungus by scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) revealed the presence of secondary protein structures. Furthermore, this study demonstrated that the application of ragi husk could be a promising feedstock for value-added industrial products. The thermo-acidophilic nature of isolated strain Aspergillus fumigatus JCM 10253 possessed a significant potential for higher titer of cellulase production that could be further employed for lignocellulosic bioethanol production.





Similar content being viewed by others
References
Zabed H, Faruq G, Sahu JN, Azirun MS, Hashim R, Nasrulhaq Boyce A (2014) Bioethanol production from fermentable sugar juice. Sci World J. 2014:11. https://doi.org/10.1155/2014/957102
Balan V (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN biotechnol. 2014:31. https://doi.org/10.1155/2014/463074
Latifian M, Hamidi-Esfahani Z, Barzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Biores Technol 98:3634–3637
Medina-Morales M, Martínez-Hernández JL, De La Garza H, Aguilar CN (2011) Cellulolytic enzymes production by solid state culture using pecan nut shell as substrate and support. AJABS 6:196–200
El-Shishtawy RM, Mohamed SA, Asiri AM, Abu-bakr MG, Ibrahim IH, Al-Talhi HA (2014) Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megatherium. BMC Biotechnol 14:29
Arora R, Behera S, Sharma NK, Singh R, Yadav YK, Kumar S (2014) Biochemical conversion of rice straw (Oryza sativa L.) to bioethanol using thermotolerant isolate K. marxianus NIRE-K3. In: Proceedings of exploring and basic sciences for Next Generation Frontiers. Elsevier, New Delhi, pp 143–146.
Mallerman J, Papinutti L, Levin L (2015) Characterization of β-glucosidase produced by the white rot fungus Flammulina velutipes. J Microbiol Biotechnol 25:57–65
Andrade CM, Pereira N Jr, Antranikian G (1999) Extremely thermophilic microorganisms and their polymer-hidrolytic enzymes. Rev Microbiol 30:287–298
Saroj P, Manasa P, Narasimhulu K (2018) Characterization of thermophilic fungi producing extracellular lignocellulolytic enzymes for lignocellulosic hydrolysis under solid-state fermentation. Bioresour Bioprocess 5:31
Bellundagi V, Umesh KB, Ravi SC (2016) Growth dynamics and forecasting of finger millet (ragi) production in Karnataka. Econ Aff 61(2):195–201
Jampala P, Tadikamalla S, Preethi M, Ramanujam S, Uppuluri KB (2017) Concurrent production of cellulase and xylanase from Trichoderma reesei NCIM 1186: enhancement of production by desirability-based multi-objective method. 3 Biotech 7:14
Srikanth R, Siddartha G, Reddy CHS, Harish B, Ramaiah MJ, Uppuluri KB (2015) Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohyd Polym 123:8–16
Ahamed A, Vermette P (2008) Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J 40:399–407
Ahamed A, Vermette P (2010) Effect of mechanical agitation on the production of cellulases by Trichoderma reesei RUT-C30 in a draft-tube airlift bioreactor. Biochem Eng J 49:379–387
Shajahan S, Moorthy IG, Sivakumar N, Selvakumar G (2017) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra, India. J King Saud Univ Sci 29:302–310
Kumar A, Gautam A, Dutt D (2016) Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a cost-effective method to produce cellulases for the hydrolysis of pearl millet stover. Fermentation 2:12
Mandels M, Weber J (1969) Prod Cellul ACS Publ 95:391–414
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Grover AK, MacMurchie DD, Cushley RJ (1977) Studies on almond emulsin β d-glucosidase I. Isolation and characterization of a bifunctional isozyme. Biochim et Biophys Acta (BBA)-Enzymol 482:98–108
Kovács K, Szakacs G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broths and washed mycelia of Trichoderma reesei and Trichoderma atroviride. Biores Technol 100:1350–1357
Zakaria B, Nassar H, Amr SA, El-Gendy NS (2015) Applying factorial design and response surface methodology to enhance microbial denitrogenation by tween 80 and yeast extract. Pet Sci Technol 33:880–892
Bai H, Wang H, Junde Sun MI, Han M, Huang Y, Han X, Yang Q (2013) Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation. EXCLI J 12:528
Manan M (2017) The morphology and structure of red pigment producing fungus: Monascus purpureus. J Microbiol Exp 5:00138
González-Ramírez AI, Ramírez-Granillo A, Medina-Canales MG, Rodríguez-Tovar AV, Martínez-Rivera MA (2016) Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol 16:243
Ramage G, Rajendran R, Gutierrez-Correa M, Jones B, Williams C (2011) Aspergillus biofilms: clinical and industrial significance. FEMS Microbiol Lett 324:89–97
Villena G, Gutiérrez-Correa M (2007) Morphological patterns of Aspergillus niger biofilms and pellets related to lignocellulolytic enzyme productivities. Lett Appl Microbiol 45:231–237
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25:1–30
Herculano PN, Porto TS, Moreira KA, Pinto GA, Souza-Motta CM, Porto ALF (2011) Cellulase production by Aspergillus japonicus URM5620 using waste from castor bean (Ricinus communis L.) under solid-state fermentation. Appl Biochem Biotechnol 165:1057–1067
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev 64:461–488
Olajuyigbe FM, Nlekerem CM, Ogunyewo OA (2016) Production and characterization of highly thermostable β-glucosidase during the biodegradation of methyl cellulose by Fusarium oxysporum. Biochem Res Int. https://doi.org/10.1155/2016/3978124
Gautam S, Bundela P, Pandey A, Khan J, Awasthi M, Sarsaiya S (2011) Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol Res Int. https://doi.org/10.4061/2011/810425
Liu D, Zhang R, Yang X, Wu H, Xu D, Tang Z, Shen Q (2011) Thermostable cellulase production of Aspergillus fumigatus Z5 under solid-state fermentation and its application in degradation of agricultural wastes. Int Biodeterior Biodegrad 65:717–725
Soni R, Nazir A, Chadha B (2010) Optimization of cellulase production by a versatile Aspergillus fumigatus fresenius strain (AMA) capable of efficient deinking and enzymatic hydrolysis of solka floc and bagasse. Ind Crops Prod 31:277–283
Mehboob N, Asad MJ, Asgher M, Gulfraz M, Mukhtar T, Mahmood RT (2014) Exploring thermophilic cellulolytic enzyme production potential of Aspergillus fumigatus by the solid-state fermentation of wheat straw. Appl Biochem Biotechnol 172:3646–3655
Mallek-Fakhfakh H, Fakhfakh J, Masmoudi N, Rezgui F, Gargouri A, Belghith H (2017) Agricultural wastes as substrates for β-glucosidase production by Talaromyces thermophilus: role of these enzymes in enhancing waste paper saccharification. Prep Biochem Biotechnol 47:414–423
Bharti AK, Kumar A, Kumar A, Dutt D (2018) Exploitation of Parthenium hysterophorous biomass as low-cost substrate for cellulase and xylanase production under solid-state fermentation using Talaromyces stipitatus MTCC 12687. J Radiat Res Appl Sci 11:271–280
Richard TL, Veeken AH, De Wilde V, Hamelers H (2004) Air-filled porosity and permeability relationships during solid-state fermentation. Biotechnol Prog 20:1372–1381
Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y (2008) Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Biores Technol 99:7623–7629
Sandhya C, Sumantha A, Szakacs G, Pandey A (2005) Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem 40:2689–2694
Gautam A, Kumar A, Dutt D (2015) Production of cellulase-free xylanase by Aspergillus flavus ARC-12 using pearl millet stover as the substrate under solid-state fermentation. J Adv Enzym Res 1:1–9
Kumar B, Bhardwaj N, Alam A, Agrawal K, Prasad H, Verma P (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB EXPRESS 8:173
Narasimha G, Sridevi A, Buddolla V, Subhosh CM, Rajasekhar RB (2006) Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr J Biotech 5:472–476
Akula S, Golla N (2018) Optimization of cellulase production by Aspergillus niger isolated from forest soil. Open Biotechnol J 12:256–269
Fernandes TG, López JA, Silva LA, Polizeli MdLT, Silva DP, Ruzene DS, Carvalho ML, Carvalho ÍF (2018) Prospecting of soybean hulls as an inducer carbon source for the cellulase production. Prep Biochem Biotechnol 48:743–749
Romero M, Aguado J, González L, Ladero M (1999) Cellulase production by Neurospora crassa on wheat straw. Enzyme and Microb Technol 25:244–250
Xia L, Cen P (1999) Cellulase production by solid state fermentation on lignocellulosic waste from the xylose industry. Process Biochem 34:909–912
Grigorevski-Lima A, Da Vinha F, Souza D, Bispo A, Bon E, Coelho R, Nascimento R (2009) Aspergillus fumigatus thermophilic and acidophilic endoglucanases. Appl Biochem Biotechnol 155:18–26
El-Nahrawy S, Metwally M, El-Kodoos A, Rizk Y, Belal E-SB, Shabana SA, El-Refai IM (2017) Optimization of culture conditions for production of cellulase by Aspergillus tubingensis KY615746 using rice straw waste. Environ Biodivers Soil Security 1:177–189
Xu X, Lin M, Zang Q, Shi S (2018) Solid state bioconversion of lignocellulosic residues by Inonotus obliquus for production of cellulolytic enzymes and saccharification. Biores Technol 247:88–95
Delabona PS, Pirota RDPB, Codima CA, Tremacoldi CR, Rodrigues A, Farinas CS (2013) Effect of initial moisture content on two Amazon rainforest Aspergillus strains cultivated on agro-industrial residues: biomass-degrading enzymes production and characterization. Ind Crops Prod 42:236–242
Silva VCT, Coto ALS, Souza RC, Neves MBS, Gomes E, Bonilla-Rodriguez GO (2016) Effect of pH, temperature, and chemicals on the endoglucanases and β-glucosidases from the thermophilic fungus Myceliophthora heterothallica F.2.1.4. obtained by solid-state and submerged cultivation. Biochem Res Int. https://doi.org/10.1155/2016/9781216
Saini A, Aggarwal NK, Yadav A (2017) Cost-effective cellulase production using Parthenium hysterophorus biomass as an unconventional lignocellulosic substrate. 3 Biotech 7:12
Sun Y-X, Shen B-B, Han H-Y, Lu Y, Zhang B-X, Gao Y-F, Hu B-Z, Hu X-M (2018) Screening of potential IL-tolerant cellulases and their efficient saccharification of IL-pretreated lignocelluloses. RSC Adv 8:30957–30965
Preetha B, Viruthagiri T (2007) Application of response surface methodology for the biosorption of copper using Rhizopus arrhizus. J Hazard Mater 143:506–510
Sherief A, El-Tanash A, Atia N (2010) Cellulase production by Aspergillus fumigatus grown on mixed substrate of rice straw and wheat bran. Res J Microbiol 5:199–211
Sarkar N, Aikat K (2014) Aspergillus fumigatus NITDGPKA3 provides for increased cellulase production. Int J Chem Eng. 2014:1–9. https://doi.org/10.1155/2014/959845
Gilna V, Khaleel K (2011) Cellulase enzyme activity of Aspergillus fumigatus from mangrove soil on lignocellulosic substrate. Recent Res Sci Technol 3:132–134
Elsa C, Kumar M, Baskar G (2015) Optimized production of cellulase using fruit waste and its application in bioethanol production. Int J Pharma Bio Sci 6(2):1005–1013
Bagewadi ZK, Ninnekar HZ (2015) Production, purifiation and characterization of endoglucanase from Aspergillus fumigatus and enzymatic hydrolysis of lignocellulosic waste. Int J Biotechnol Biomed Sci 1:25–32
Ang S, Shaza E, Adibah Y, Suraini A, Madihah M (2013) Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem 48:1293–1302
Mohapatra S, Padhy S, Mohapatra PKD, Thatoi H (2018) Enhanced reducing sugar production by saccharification of lignocellulosic biomass, Pennisetum species through cellulase from a newly isolated Aspergillus fumigatus. Biores Technol 253:262–272
Krimm S, Bandekar J (1986) Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein chem Elsevier.
Bai H, Wang H, Sun J, Irfan M, Han M, Huang Y, Han X, Yang Q (2013) Purification and characterization of beta 1,4-glucanases from Penicillium simplicissimum H-11. BioResources 8:3657–3671
Acknowledgments
The authors acknowledge the facility provided by SAIF, IIT Bombay for FTIR analysis, and also the support provided by the Department of Biotechnology, National Institute of Technology Warangal, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saroj, P., P, M. & Narasimhulu, K. Assessment and evaluation of cellulase production using ragi (Eleusine coracana) husk as a substrate from thermo-acidophilic Aspergillus fumigatus JCM 10253. Bioprocess Biosyst Eng 44, 113–126 (2021). https://doi.org/10.1007/s00449-020-02428-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02428-z