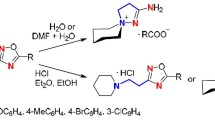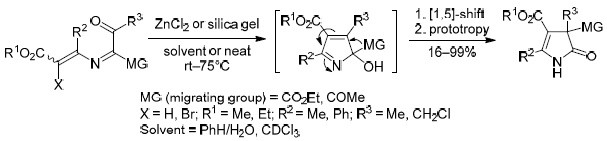
1-Acyl-2-azabuta-1,3-dienes underwent rearrangement to 4-pyrrolin-2-ones in the presence of acidic catalysts (silica gel or ZnCl2) and water. The reaction proceeded via the formation of 2,3-dihydroxy-3,4-dihydropyrrole and 2-hydroxy-2Н-pyrrole intermediates, the latter of which was transformed by [1,5]-sigmatropic shift of carbonyl substituent and prototropic isomerization. 4-Chloro-substituted 1-acetyl-2-azabuta-1,3-dienes were stable toward rearrangement, while brominated analogs rearranged into 4-pyrrolin-2-ones, probably as a result of the initial radical hydrodebromination. According to calculations performed at the DFT level, two steps of the reaction were catalyzed by acid: the cyclization of 2-azabutadiene and the sigmatropic shift in the 2-hydroxy-2Н-pyrrole intermediate.

Similar content being viewed by others
References
(a) Monbaliu, J.-C. M.; Masschelein, K. G. R.; Stevens, C. V. Chem. Soc. Rev. 2011, 40, 4708. (b) Jayakumar, S.; Ishar, M. P. S.; Mohinder, P.; Mahajan, M. P. Tetrahedron 2002, 58, 379.
Shao, X.; Malcolmson, S. J. Org. Lett. 2019, 21, 7380.
Golubev, A. A.; Smetanin, I. A.; Agafonova, A. V.; Rostovskii, N. V.; Khlebnikov, A. F.; Starova, G. L.; Novikov, M. S. Org. Biomol. Chem. 2019, 17, 6821.
Vargas, D. F.; Larghi, E. L.; Kaufman, T. S. Nat. Prod. Rep. 2019, 36, 354.
Smetanin, I. A.; Novikov, M. S.; Rostovskii, N. V.; Khlebnikov, A. F.; Starova, G. L.; Yufit, D. S. Tetrahedron 2015, 71, 4616.
(a) Smetanin, I. A.; Novikov, M. S.; Agafonova, A. V.; Rostovskii, N. V.; Khlebnikov, A. F.; Kudryavtsev, I. V.; Terpilowski, M. A.; Serebriakova, M. K.; Trulioff, A. S.; Goncharov, N. V. Org. Biomol. Chem. 2016, 14, 4479. (b) Koronatov, A. N.; Rostovskii, N. V.; Khlebnikov, A. F.; Novikov, M. S. Chem. Heterocycl. Compd. 2019, 55, 1185. [Khim. Geterotsikl. Soedin. 2019, 55, 1185.]
Rostovskii, N. V.; Novikov, M. S.; Khlebnikov, A. F.; Khlebnikov, V. A.; Korneev, S. M. Tetrahedron 2013, 69, 4292.
Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Yufit, D. S. Tetrahedron 2013, 69, 4546.
(a) Montagnon, T.; Kalaitzakis, D.; Sofiadis, M.; Vassilikogiannakis, G. Org. Biomol. Chem. 2020, 18, 180. (b) Ba, D.; Chen, Y.; Lv, W.; Wen, S.; Cheng, G. Org. Lett. 2019, 21, 8603. (c) Gao, P.; Wang, J.; Bai, Z.; Fan, M.-J.; Yang, D.-S.; Guan, Z.-H. Org. Lett. 2018, 20, 3627. (d) Huang, H.; Hu, B.; Lai, Y.; Zou, Z.; Lin, H.; Xiao, Y.; You, Q.; Shen J. Adv. Synth. Catal. 2018, 360, 3906.
(a) Kong, C.; Driver, T. G. Org. Lett. 2015, 17, 802. (b) Kawashima, K.; Hiromoto, M.; Hayashi, K.; Kakehi, A.; Shiro, M.; Noguchi, M. Tetrahedron Lett. 2007, 48, 941. (c) Poriel, C.; Lachia, M.; Wilson, C.; Davies, J. R.; Moody, C. J. J. Org. Chem. 2007, 72, 2978. (d) Schenck, L. W.; Sippel, A.; Kuna, K.; Frank, W.; Albert, A.; Kucklaender, U. Tetrahedron 2005, 61, 9129. (e) Acheson, R. M. Acc. Chem. Res. 1971, 4, 177.
Moon, M. W. J. Org. Chem. 1977, 42, 2219.
Kovach, J.; Brennessel, W. W.; Jones, W. D. RSC Adv. 2014, 4, 1401.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
This work received financial support from the Russian Science Foundation (Project No. 20-13-00044).
The authors would like to express their gratitude to Е. О. Kalinin (Institute of Chemistry, Saint Petersburg State University) for providing the labeled water.
The analysis of obtained compounds and quantumchemical calculations were performed at the Saint Petersburg State University resource centers “Magnetic Resonance Research Center”, “Chemical Analysis and Materials Research Center"“, “Research Center for X-ray Diffraction Studies”, “Chemistry Educational Center”, and “Computing Center”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(7), 881–887
Electronic supplementary material
ESM 1
(PDF 4797 kb)
Rights and permissions
About this article
Cite this article
Rostovskii, N.V., Smetanin, I.A., Koronatov, A.N. et al. Acid-catalyzed rearrangement of 1-acyl-2-azabuta-1,3-dienes to 4-pyrrolin-2-ones. Chem Heterocycl Comp 56, 881–887 (2020). https://doi.org/10.1007/s10593-020-02745-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02745-x