-
PDF
- Split View
-
Views
-
Cite
Cite
Karina Wieczorek, Dominik Chłond, Łukasz Junkiert, Piotr Świątek, Structure of the reproductive system of the sexual generation of the endemic Arctic species Acyrthosiphon svalbardicum and its temperate counterpart Acyrthosiphon pisum (Hemiptera, Aphididae), Biology of Reproduction, Volume 103, Issue 5, November 2020, Pages 1043–1053, https://doi.org/10.1093/biolre/ioaa147
Close - Share Icon Share
Abstract
The Arctic aphids live briefly and must breed quickly to survive. Shortened life cycle, with only two generations: the stem mother and sexuales—oviparous females and males is an adaptation for optimal use of the short breeding period, which lasts from late July to the end of August. Using Acyrthosiphon svalbardicum, an endemic High Arctic aphid species, we describe the structure of the reproductive system of sexual morphs and compare with its temperate counterparts, in particular the model organism the pea aphid Acyrthosiphon pisum. Generally, the histological composition and ultrastructure of reproductive system of sexuales of A. svalbardicum is broadly similar to the reproductive systems described already in other species of aphids. The unique characters include in both oviparous females and males an enormous layer of the fat body, adhering to the structures of the internal reproductive system. The greatly enlarged accessory glands of males accumulate a heterogenous secretion composed of irregularly organized bunches of spicule-like structures of high electron density embedded in fine and coarse granular material. This material, unknown among temperate counterparts of A. svalbardicum, during mating is transported from the accessory glands of the male to its ejaculatory duct, where it is mixed with the ejaculate, and then is transferred to the spermatheca of the oviparous female.
Introduction
The Svalbard archipelago, located from 74° to 81° north latitude and from 10° to 35° east longitude, is one of the best studied Arctic regions in terms of biodiversity, including the invertebrate fauna [1–5]. Among about 300 insect taxa reported on Svalbard [5], the order Hemiptera is represented exclusively by three native species of aphids (Aphididae): Acyrthosiphon svalbardicum Heikinheimo, Sitobion calvulus Ossiannilsson, and Pemphigus sp. All of them have highly restricted distributions, i.e., are known only from a few scattered localities; A. svalbardicum and S. calvulus are treated as endemic to Svalbard [4].
A. svalbardicum is a holocyclic, monophagous species, feeding on the eight-petal mountain-avens Dryas octopetala L. ssp. octopetala (Rosaceae), at the base of the leaves or on the flower shoots under the petals. Its distribution closely depends on various biotic and abiotic factors, i.e., not only the presence of the host plant determines its occurrence. Its spatial distribution along the west coast of Spitsbergen is very patchy at the local scale and partially determined by winter snow depth modulating the length of the summer growing season [6–8]. Due to the climatic conditions, the species could probably not establish populations beyond 79°12′, which constitutes its northernmost limit, and at the same time the most north location for any aphid [9].
In contrast to most aphid species in temperature climate, A. svalbardicum is characterized by an extremely adaptive life cycle. The stem mother (fundatrix), which emerges from the overwintering egg, gives birth directly to sexual morphs—oviparous females and males [10–13]. Only a small number of viviparous wingless or winged females, the dominant generation in temperate aphids, have been observed so far [14, 15]. Moreover, by contrast with temperate aphid species, where seasonal polyphenism is photoperiodically induced [16], this shortened life cycle is genetically controlled [11, 12] and strongly adapted to harsh environmental conditions—a short, cool summer season with sub-zero temperatures and consecutive 24-h periods of sunlight (the midnight sun or the polar day).
Due to its biology, A. svalbardicum has become a model species and the object of numerous studies concerning life strategies and ecology of terrestrial arthropods in the arctic environment [6–8, 17–19], especially in a scenario of continued climate warming [6, 7, 19–25]. However, the structure of its reproductive system has not been examined before. Because A. svalbardicum is characterized by an extremely adaptive life cycle, it is also an excellent model for understanding the development and structure of the reproductive system, as well as the reproductive strategies of these insects in the harsh conditions of the High Arctic. Thus, the aim of this paper is to describe the reproductive system of the adult wingless males and oviparous females of A. svalbardicum using light and electron microscopy. We present its morphology, histology, and ultrastructure and comparison with the reproductive system of its temperate counterparts, in particular the model organism the pea aphid Acyrthosiphon pisum Harris. The present paper fills the significant gap and complements previous research concerning this rare species of the Svalbard environment, of low dispersal, genetically isolated populations, and a highly sensitive indicator of changing climate.
Materials and methods
Acyrthosiphon svalbardicum
Study site
The field work was conducted in the vicinity of the world’s northernmost human settlements, Ny-Ålesund research stations (78°55′ N, 11°56′ E), situated in Kongsfjorden on north-western Spitsbergen, the largest of the Svalbard islands.
Prior to extending the database of collection sites of the species studied, all localities were marked in the Global Positioning System. Field photographs were taken using an iPhone 7 camera with the OlloClip Macro Pro Lens Set. The permission for the research within Management Area 10 (RIS-ID 10994) was issued by the Governor of Svalbard.
Sampling procedure
The field study was conducted from 8 to 22 August 2018. The aphids were collected directly from D. octopetala by shaking the host plants. Some plant fragments were kept warm until hidden aphids left them. Then, the aphids were placed into Eppendorf tubes containing 70% ethanol or 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Samples were identified based on their morphological diagnostic features using original descriptions of the wingless viviparous female, oviparous female and male [10, 11]. The aphid material is deposited in the collection of the Department of Zoology, University of Silesia in Katowice, Katowice, Poland (DZUS): DZUS 21/8.18_1 Acyrthosiphon svalbardicum, three apterous viviparous females, Ny-Ålesund, Norway, 21.08.2018, Dryas octopetala, K.Wieczorek leg.; DZUS 21/8.18_2 Acyrthosiphon svalbardicum, two oviparous females, Ny-Ålesund, Norway, 21.08.2018, Dryas octopetala, K.Wieczorek leg.; DZUS 21/8.18_3 Acyrthosiphon svalbardicum, two apterous males, Ny-Ålesund, Norway, 21.08.2018, Dryas octopetala, K.Wieczorek leg.
Light, transmission, and scanning electron microscopy
In laboratory conditions, the reproductive system (from 3 males and 15 oviparous females) was dissected and examined using a Nikon SMZ 25 stereoscopic microscope and photographed using a Nikon DS-Fi2 camera. The drawings of the reproductive system were made freehand on the Nikon Ni-U light microscope using a camera lucida. The protocol for the scanning electron microscopic study of the male genitalia (two individuals) followed the method described by Wieczorek et al. [26]. For the histological and ultrastructural studies, the insects (3 males, 21 oviparous females, and 2 pairs in copula) were decapitated and fixed again in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4 for a week. After washing in phosphate buffer (pH 7.4), the material was post-fixed for 2 h in 1% OsO4 in phosphate buffer, pH 7.4. The post-fixed material was washed in a graded series of ethanol, which was replaced with acetone and embedded in an Epoxy Embedding Medium Kit (Sigma, St. Louis, MO, USA). Semi-thin sections (0.7 μm thick) were cut on a Leica Ultracut ultramicrotome and stained with 1% methylene blue in a 1% sodium biborate solution at room temperature for 30 s. In addition, semi-thin sections were stained using the periodic acid Schiff (PAS) method to localize the polysaccharides and bromophenol blue for the polypeptides. All of the sections were examined under an Olympus BX60 microscope equipped with an XC50 digital camera (Olympus, Tokyo, Japan) and cellSens Standard software (Olympus, Tokyo, Japan). Ultra-thin sections (80 nm) were cut on an RMC Power XT ultramicrotome (RMC Boeckeler, Tucson, AZ, USA). The ultra-thin sections were contrasted with uranyl acetate (30 min) and lead citrate (20 min). The contrasted sections were examined using a Hitachi H500 transmission electron microscope at 75 kV.
Acyrthosiphon pisum
All procedures (sample collection and insect rearing, light and transmission electron microscopy) were conducted as it was previously described [26]. For comparative analysis, only sexual morphs before or after copulation were chosen.
Results
Field study
The aphids were collected in daily field trips in the vicinity of Ny-Ålesund research stations, in the relatively simple Arctic ecosystems with a patch of D. octopetala tundra, which is the defined host of A. svalbardicum. Although D. octopetala is quite a common plant in the search area, the aphids were collected only from few locations, mainly on the east slope of Brandalpynten cape, west of Ny-Ålesund (78°55′34.67″N 11°53′42.64″E; 78°55′31.67″N 11°54′13.71″E; 78°55′39.59″N 11°54′35.50″E; 78°55′34.13″N 11°55′10.68″E). Although aphids were hidden singly at the base of the leaves of their host plant, as many as 98 oviparous females and 15 males (including two pairs in copula) were found, collected, and preserved for further morphological and anatomical studies.
Morphology, histology, and ultrastructure of the male reproductive system of A. svalbardicum
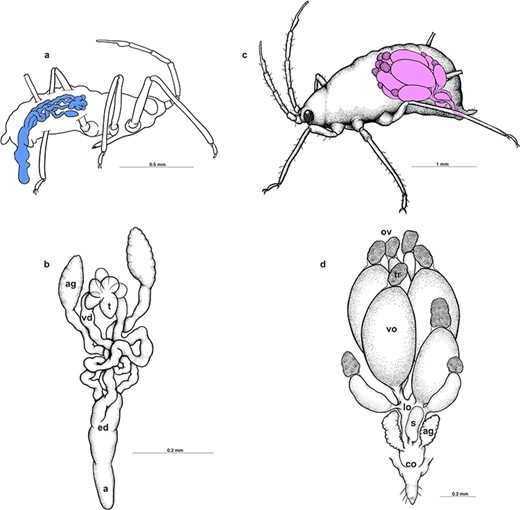
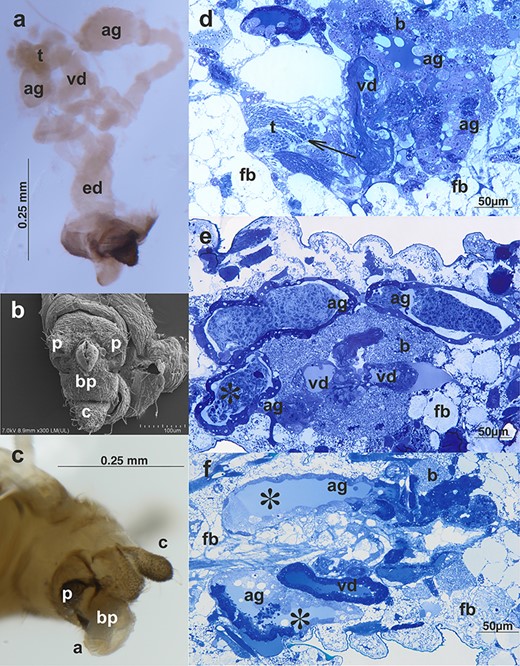
Males of A. svalbardicum are wingless, 0.92–1.36 mm long, olive green with dark green abdomen, and black antennae and legs (Figure 1a and c). The reproductive system of the male A. svalbardicum runs parallel to the longitudinal axis of the body (Figure 2a). Its components—testis, vasa deferentia, and accessory glands—closely adjoin each other (Figure 2a–b) and are surrounded by a very thick layer of cells densely packed with lipid droplets, which were interpreted as the fat body cells (Figure 3d–f). Testis follicles, three per testis, are small, rounded, and arranged in a rosette (Figures 2a–b, 3a). Vasa deferentia run separately and are strongly expanded, especially in the part nearer to the testes. Accessory glands are the dominant components of the internal reproductive system. They are enlarged with a distinct capitate extension at the end of each gland (Figures 2b, 3a). The outlets of vasa deferentia and accessory glands run separately, opening to the elongated ejaculatory duct (Figure 2b). The external genitalia are composed of paired triangular parameres covered by numerous long spine-like setae and the hook-shaped basal parts of the phallus, which is housed among them (Figure 3b) and inverted during mating (Figures 1c, 3c).
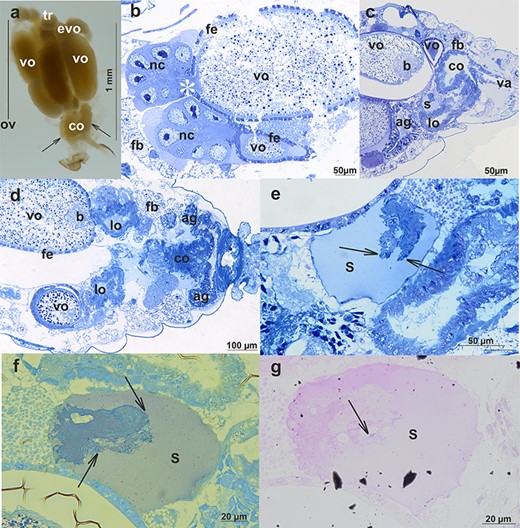
Sexual generation of A. svalbardicum: (a) wingless male, (b) oviparous female, and (c) copulating pair of sexuales.
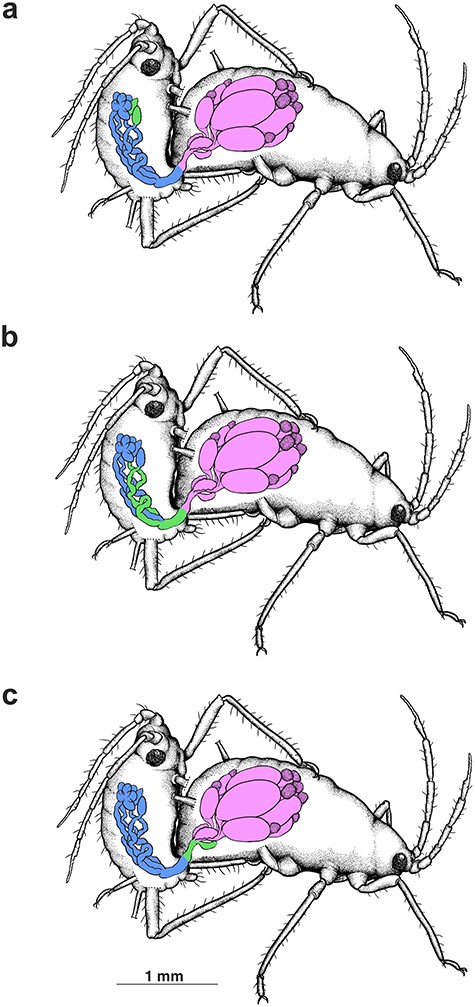
Schematic drawings presenting the localization (a and c) and morphology (b and d) of the reproductive system of male and oviparous female, respectively. ag, accessory glands; co, common oviduct; ed, ejaculatory duct; lo, lateral oviduct; ov, ovarioles; a, aedeagus; s, spermatheca; t, testes; tr, tropharia; vd, vasa deferentia; vo, vitellogenic oocyte.
Male reproductive system of A. svalbardicum: (a) whole system visualized by a stereomicroscope, ag, accessory glands; ed, ejaculatory duct; t, testes; vd, vasa deferentia; (b) scanning electron microscopy and (c) light microscopy of the male external genitalia, a, aedeagus; bp, basal parts of phallus; c, cauda; p, parameres; (d and e) a specimen before copulation—histological sections through the abdomen; (f) a section through the abdomen of a male during copulation. Testes (t) with visible bunches of spermatozoa (arrow), ag, accessory gland; vd, vas deferens; ed, ejaculatory duct; b, bacteria; and fb, fat body. Note the huge accumulations of secretion within accessory glands in the male before copulation (asterisks in e) and the lack of such accumulations in the specimen during copulation (asterisks in f). Light microscopy, Epon semi-thin sections stained with methylene blue.
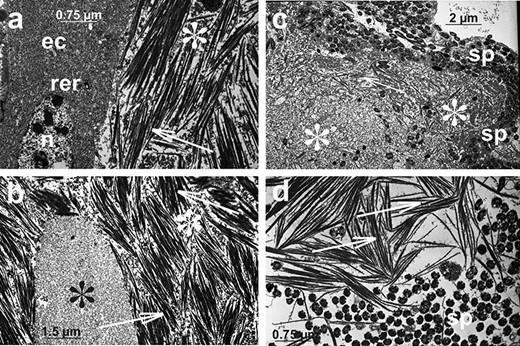
The histological composition and ultrastructure of the male reproductive system of A. svalbardicum is broadly similar to the male reproductive systems described already in other males of aphids, especially to the system recently analyzed in detail in A. pisum [26]. However, some specific components were also found. Briefly, all internal components (i.e., testicular follicles, vasa deferentia, accessory glands, and ejaculatory duct) are made up of flattened or cuboidal epithelial cells standing on the thin basal lamina and encompassed by poorly developed muscle strands (Figures 3d–f, 4a). Within testicular follicles of males before copulation, bunches of ripe spermatozoa (Figure 3d) or even late spermatids (not shown) were observed. In specimens during and after copulation spermatozoa within testicular follicles were not observed. The epithelial cells of vasa deferentia and accessory glands show morphology that is consistent with high secretory activity. These cells are filled with numerous cisternae of rough endoplasmic reticulum (Figure 4a) and within the lumen of both structures a secretion can be found (Figure 3d–f). This secretion is usually heterogeneous, i.e., it has the form of a liquid substance, which is moderately stained with methylene blue and fills the lumen ducts (Figure 3d–f) and within it, especially in the case of accessory glands in males before copulation, accumulations of solid secretion can be observed (Figure 3e). Ultrastructural analysis of this solid secretion found within the accessory glands of males before copulation revealed that it is composed of fine fibrillar material of high electron density usually organized in more or less parallelly orientated bunches of spicule-like morphology (Figure 4a–b). These spicule-like bunches are embedded in fine or more coarse and solid granular material suspended in the liquid content of accessory gland (Figure 4a–b). Granular material seems to be suspended in the liquid content of accessory gland. Singular fibrils visualized on ultra-thin sections can be 3.8 μm long (the average length on sections is 3.5 μm, n = 5) and its average diameter is 38.2 nm (n = 5), whereas bunches can reach thickness of 1.8 μm (measured from ultra-thin sections, the average thickness is 1.5 μm, n = 5). In accessory glands of males during and after copulation liquid substance was only observed (Figure 3f). The solid fraction was absent, but ultrastructural identical solid fraction was observed within the spermatheca of females during and after copulation (see the next section). Within vasa deferentia of males before copulation spermatozoa can be observed (Figure 3d), whereas in a specimen during copulation spermatozoa were observed both in vasa deferentia and in the ejaculatory duct (not shown). In males after copulation singular spermatozoa were found within vasa deferentia and/or the ejaculatory duct. The ejaculatory duct is built of flattened epithelial cells standing on thin basal lamina. The apical parts of these epithelial cells are covered by a cuticle and encompassed by longitudinal and circular muscles (not shown).
Ultrastructural details of the accessory gland secretion of the male A. svalbardicum before and after copulation. (a and b) A fragment of accessory gland in males before copulation (compare with Figure 3e). Epithelial cell (ec) is filled with rough endoplasmic reticulum (rer); n, nucleus. Glands are filled with spicule-like inclusions (arrows) and granular material of various electron density and grain dimensions (fine granular, black asterisks, and more coarse, white asterisks). (c and d) Material deposited within spermatheca lumen in females after copulation (compare with Figure 5e–f). Spermatozoa (sp), spicule-like inclusions (arrows), and granular material (asterisks) are visible. Transmission electron microscopy.
Morphology, histology, and ultrastructure of the oviparous female reproductive system of A. svalbardicum
Oviparous females of A. svalbardicum are 1.06–1.57 mm long and dark green with antennae and legs paler; yellowish ovaries are visible in the middle, transparent part of the abdomen (Figure 1b–c). Ovaries, composed of eight ovarioles each, tightly fill the abdomen (Figure 2c) and are surrounded by a very thick layer of the fat body (Figure 5b–d). Ovaries are connected via lateral oviducts with a common oviduct, whereas paired accessory glands and an unpaired spermatheca are located laterally to them (Figure 2d). In general, histology and ultrastructure of ovaries and reproductive tracts in the species studied are similar to the female reproductive organs described recently in oviparous females of A. pisum [26]. Ovarioles, as in other aphids and more generally in all hemipterans, are of telotrophic meroistic type (Figures 2d, 5a). It means that the apical part of each ovariole is occupied by a tropharium (trophic chamber) made up of highly polyploid trophocytes (nurse cells) (Figures 2d, 5a–b). The rest of the ovariole is taken up by growing oocytes (previtellogenic and vitellogenic) (Figures 2d, 5a–d). Oocytes gradually take in yolk proteins and grow considerably, and in late vitellogenetic oocytes the symbiotic bacteria form a characteristic “symbiotic ball” at the posterior pole of oocytes (Figure 5c–d). Reproductive tracts, i.e., lateral and common oviducts, are built from cuboidal or columnar epithelial cells (Figure 5c–d) standing on the thin basal lamina and loosely associated muscular fibers (not shown). The accessory gland wall is formed by columnar epithelial cells (Figure 5c–d), and the apical surface of epithelial cells is covered by a thin cuticle. Accessory glands in analyzed females contained a small amount of homogeneous liquid substance of moderate density (Figure 5d). The spermatheca wall is composed of thin, flat epithelial cells (Figure 5e), covered by a thin layer of cuticle. The lumen of the spermatheca in oviparous females after copulation is filled with a homogeneous liquid substance of moderate density and with a denser, heterogeneous, and solid material of irregular shape (Figure 5c and e–g). Ultrastructural analysis of this dense material revealed that it is composed of spermatozoa associated with both amorphous material of medium electron density and high electron density fibrillar material organized in spicule-like bunches (Figure 4c–d). Morphologically, this material associated with sperm, especially spicule-like inclusions, is very similar to material found in male accessory glands in specimens before copulation (Figure 4a–b). Singular fibrils visualized on ultra-thin sections can be 4.2 μm long (the average length on sections is 3.3 μm, n = 5) and its average diameter is 44.5 nm (n = 5). Histochemical analysis showed that this substance associated with spermatozoa is slightly enriched in proteins (Figure 5f) and is weakly PAS positive (Figure 5g).
Oviparous female reproductive system of A. svalbardicum: (a) whole system visualized by a stereomicroscope. Four ovarioles are well visible (one is marked, ov)—each one is composed of a tropharium (tr) and growing oocytes—early vitellogenic (evo) and/or vitellogenic (vo). Common oviduct (co) and accessory glands (arrows) are also visible. Stereomicroscope. (b–d) Reproductive system visualized by semi-thin sections; (b) within ovarioles nurse cells (nc), nutritive cord (asterisks) and vitellogenic oocytes (vo) enveloped by follicular epithelium (fe) are visible, fb, fat body; (c and d) organization of reproductive tracts. ag, accessory glands; lo, lateral oviducts; co, common oviduct; va, vagina; and s, spermatheca are visible. In addition, vitellogenic oocytes (vo) with a symbiont ball (b), follicular epithelium (fe), and fat body (fb) can be seen. Light microscopy, Epon semi-thin sections stained with methylene blue. (e–g) Spermatheca (s) of a female after copulation is partially filled with a mixture composed of spermatozoa and male accessory gland secretion (compare with Figure 3). Spermatozoa (arrows) are stained more intensively than the accessory gland secretion. (e) Methylene blue staining, (f) bromophenol blue staining, and (g) PAS staining. Light microscopy, Epon semi-thin sections.
Schematic drawing presenting the movement of accessory gland secretion of the male A. svalbardicum during mating is presented on Figure 6.
Schematic drawing presenting the movement of accessory gland secretion of the male A. svalbardicum during mating: (a) before and in the initial phase of copulation accessory glands of male are filled with high electron density material including spicule-like structures (green); (b) during copulation this material is transported from the accessory glands of the male to its ejaculatory duct (green), where it is mixed with sperm and then (c) is transferred to the spermatheca (green) of the oviparous female.
Selected features of male and oviparous female reproductive systems of A. pisum in comparison with A. svalbardicum
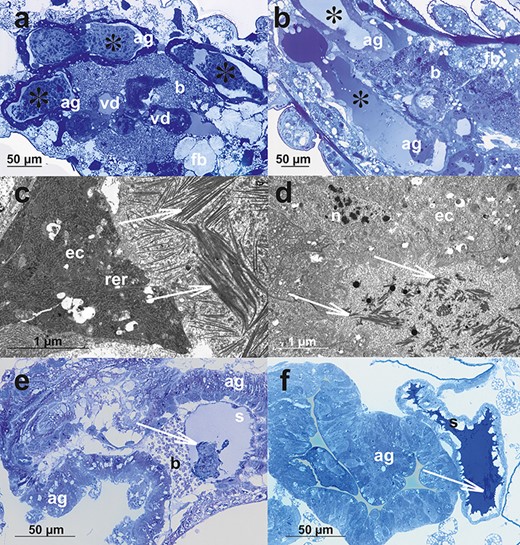
The detailed histological and ultrastructural description of reproductive systems of sexual morphs of A. pisum has been presented recently [26]. When the male accessory glands and spermatheca of A. pisum [26] and A. svalbardicum (this study) are compared, both similarities and differences were noted (Figure 7a–f). Similarly, the accessory glands of A. svalbardicum (Figures 3e and f, 7a and c) and A. pisum are composed of a layer of more or less cuboid epithelial cells of secretory character [26] (Figure 7b and d). Contrary to A. svalbardicum, in males before copulation where a mass of spicule-like bunches filled the accessory gland lumen (Figures 3e, 4a and b, 7a), in A. pisum accessory glands contain mainly substance of liquid appearance, which is slightly stained with methylene blue (Figure 7b) and is also protein positive [26]. In accessory glands of A. pisum the electron-dense fraction of secretion was also found in form of electron-dense filaments [26] (Figure 7d). However, the huge accumulations of spicule-like material have never been observed. The spermatheca of A. pisum females being after copulation contains a dense protein-positive substance [26] in which spermatozoa are embedded [26] (Figure 7f). Accumulations of high electron-dense spicule-like material characteristic for A. svalbardicum (Figures 5c and e–g, 7e) have never been observed [26] (Figure 7f).
The comparison of selected histological and ultrastructural characters of male and oviparous female reproductive systems of A. svalbardicum (a, c, e) and A. pisum (b, d, f). (a and b) Accessory glands of males before copulation; note huge aggregations of secretion with A. svalbardicum glands (asterisks in a, the same picture is presented as Figure 3e), whereas accessory glands of A. pisum (asterisks in b) contain liquid secretion differently stained with methylene blue. (c and d) Ultrastructural details of accessory glands in males before copulation. In A. svalbardicum accessory gland lumen, bunches of secretion in form of spicule-like inclusions embedded in granular material can be seen (arrows in c), whereas in A. pisum, the gland lumen is filled mainly with granular material in which some more electron-dense filaments are embedded (arrows in d). (e and f) Oviparous female accessory glands and spermathecae in specimens after copulation. A. svalbardicum spermatheca contains dense aggregation of spermatozoa and male accessory gland secretion embedded in liquid and less intensively stained secretion (arrow in e), whereas A. pisum spermatheca is packed with intensively stained secretion in which some sperm bunches are embedded (arrow in f). ag, accessory glands; b, bacteria within bacteriocytes; ec, accessory gland epithelial cells; rer, rough endoplasmic reticulum; n, nucleus; s, spermatheca. (a, b, e, f) Light microscopy, Epon semi-thin sections stained with methylene blue; (c and d) transmission electron microscopy.
Discussion
The adaptations of Arctic insects to the extreme severity and seasonality of the climatic conditions include morphological, behavioral, and physiological changes, which should be treated as complex [27]. A reduction in body size, melanism, and reduction or loss of wings are among the morphological adaptations, whereas behavioral adaptations include seeking protection, warm microhabitats, and synchronized reproduction. Physiological and biochemical changes, known as cold hardiness, include both freeze-avoidance strategies and widespread tolerance to freezing and extreme supercooling ability with synthesis of ice nucleating agents, cryoprotectants or antifreeze proteins. Other adaptations respond to prolonged or abbreviated life cycles [4, 13, 23–25, 27–29].
In the case of A. svalbardicum, morphological and behavioral adaptations are obvious. All known morphs of this species are small (the largest are stem mothers, 1.23–1.74 mm long, whereas the smallest are males, 0.92–1.36 mm long [11], present study) and dark colored. Among aphids, this species is an example of the extremely shortened life cycle with only two generations, parthenogenetic (fundatrix), followed by sexual (oviparous females and males) within the season. A similarly shortened life cycle was observed for a species of the genus Israelaphis Essig, living in different but also extreme conditions of the Mediterranean climate. Although a generation of wingless viviparous females is present in the life cycle of this genus, winged morphs are apparently very rare and the whole cycle is completed by the laying of eggs by oviparous females in spring [30]. In aphids distributed in the temperate climate regions, stem mothers and sexuales appear in a short time (stem mothers in early spring, sexuales in autumn) and an incomparably smaller number in comparison with the viviparous generations of the wingless and winged viviparous females [31]. In contrast, in the life cycle of the High Arctic aphid species, those morphs are dominant. Winged and wingless parthenogenetic females were observed for the endemic species of Svalbard aphids rarely [14]. However, both A. svalbardicum and S. calvulus respond rapidly and positively to increased summer temperature, producing these morphs [6, 7, 14, 15, 32].
In the sexual generations of these two species, the sex ratio is strongly female biased as a result of local mate competition and, due to the restricted distributions, inbreeding is almost certain [11, 32]. The sex ratio for oviparous females to males in S. calvulus was estimated as 3:1 [32]. According to our studies, the proportions of oviparous females and males of A. svalbardicum in late August, at the end of the breeding period and the aphids reproductive activity, was even lower (one male per six females). It would agree with low dispersal of this aphid species and its special sensitivity to environmental factors, in particular to patches of its host plant, the eight-petal mountain-avens.
D. octopetala-dominated plant communities are common and broadly distributed on Svalbard, especially at the dry, alkaline coastal ridges [33]. This plant is part of a typical polar semi-desert community, widespread in the patches with snow clearance early in the season. Its associated aphid species A. svalbardicum prefers to feed on flowering shoots, probably due to the availability of high-nutrient phloem sap [4]. Aphids can feed in this way only during the flowering period of the host plants, which is significantly shorter than the aphid life cycle. The field study of Ávila-Jiménez and Coulson [8], performed in early August, showed that all the life stages of the aphid occur on shoots with seed heads or flower buds rather than on vegetative shoots. Our observations, conducted in late August, revealed that the inflorescences have withered, thus sexuales of A. svalbardicum live singly, hidden at the base of the leaves of their host plant. As D. octopetala is a dwarf shrub species, forming an evergreen mat, in conditions of decreasing temperature such stable microhabitats are conducive to the continuation of the aphid life cycle, which is a significant behavioral adaptation of the species studied. It is confirmed, as the eggs were often found on the underside of the leaves on mountain-avens distributed mostly on those areas showing shallower snow depths in winter [8].
Each ovipara produced up to seven eggs (black, shiny, and turgid if fertile) of average density of 496 ± 114 eggs per square meter. Overwintering eggs are the surviving forms of holocyclic aphids in any climatic conditions. However, these eggs of A. svalbardicum are extremely cold tolerant, with a mean supercooling point (SCP) of between −36.2°C and − 38.2°C, dependent on acclimation temperature [7, 34]. Similarly, an SCP for overwintering eggs of S. calvulus ranging from −29°C to −40°C was found. Thus, for both species it was lower than the extreme winter minimum temperature recorded (−28°C) in the area of the Svalbard archipelago studied [32].
In comparison with other arthropods [27, 35, 36], physiological or biochemical adaptations to cold resistance have not yet been observed in adult aphids found in the Arctic. However, it seems that such adaptations can be observed in the structure of the reproductive system of A. svalbardicum. In particular, in comparison with other aphid species studied so far, in both oviparous females and males an enormous layer of fat body adhering to the structures of the internal reproductive system is present. In insects, the fat body is a multifunctional organ that plays a major role in the metabolism, storing, and releasing energy needed during extended non-feeding periods and for growth and reproduction [37, 38]. Storage of fatty acids and glucose is essential in insects for other functions as well. Glucose, e.g., is used for the synthesis of sugar alcohols, which are needed for adaptation to cold [37]. In A. svalbardicum, fat body cells are filled with lipid droplets and this tissue forms lobes that tightly surround internal organs. Such organization and localization of fat body is typical for insects [38]. Moreover, in aphids the primary endosymbiotic bacterium Buchnera aphidicola is harbored in specialized host cells called bacteriocytes (or mycetomes) within special organs (bacteriomes) in the fat body [39]. Arctic aphids, despite not overwintering as adults, are also exposed to significant temperature fluctuations during the short, cold summer. Comparison of the air temperature of Kongsfjorden, the main area of A. svalbardicum distribution, from the last 40 years showed that the yearly mean air temperature in Ny-Ålesund for the period from 1975 to 2000 was −5.8°C. July was the warmest month, with a mean temperature of 4.9°C, whereas February was the coldest, with a mean temperature of −14.2°C. Over the period from 2005 to 2016 in Ny-Ålesund, the multiannual yearly mean air temperature was −3.43°C, with the highest temperature recorded in July (5.9°C) and the lowest recorded in March (−9.9°C) [40]. Although comparison of the air temperature of Kongsfjorden from the last 40 years showed that the mean annual air temperature has increased by approximately 2°C (and it is expected that for the whole of Svalbard the temperatures will continue this tendency) [41], Arctic aphids are exposed to incomparably lower temperatures than their temperate counterparts. Thus, an extensive layer of fat body is an advantage in maintaining the appropriate level of energy demand and supporting a high level of reproduction.
Conducting research at the end of the growing season, in late August, we can confirm that in a relatively short time a representative sample of sexuales was collected, including copulating pairs of aphids. In general, the composition of the reproductive system of the sexual generation of A. svalbardicum is broadly similar to these structures described already in other aphids, and in particular with its temperate counterpart A. pisum [26, this study]. Comparing the previously studied species of aphids [26, 42–47] and the present results, we can formulate the following conclusions regarding the structure of the reproductive system of the sexual generation of aphids: (1) irrespective of the type of life cycle (heteroecious or monoecious; typical or shortened) and the size of the male (dwarfish or not), the histology of the male reproductive system of aphids is broadly similar; (2) histologically, the components of the system such as the testis follicle, vasa deferentia, and accessory glands are simple and have a more or less flattened epithelium of a secretory type and thin muscle fibers with a very similar ultrastructure; (3) as the male reproductive system of aphids is marked by the lack of a vesiculae seminalis, the epithelial cells of the walls of the vasa deferentia and the accessory glands have secretory functions; (4) the level of spermatogenesis is correlated with the age of the males and probably occurs in an earlier developmental stages; (5) the number of testis follicles is variable within taxa as the way that they fuse; however, these are quite constant in the Macrosiphini species including A. pisum and A. svalbardicum; (6) the epithelium of the ejaculatory duct is covered by a cuticle and is enveloped by a well-developed layer of muscles; (7) the ovariole structure and the course of oogenesis is similar within oviparous morphs of the aphids that have been studied to date; (8) the posterior ooplasm of the vitellogenic oocytes contains symbiotic bacteria that form a characteristic aggregation—a “symbiotic ball”; (9) the lateral and common oviducts, accessory glands, and spermatheca of oviparous females are built of epidermal cells standing on the thin basal lamina and are associated with the poorly developed musculature; (10) the oviparous female accessory glands produce a dense secretion; the cuticle covering the surface of the accessory gland epithelium has characteristic interruptions. In contrast, in A. svalbardicum some specific components, unknown in its temperate counterparts, were also found, which can combine with adaptation to harsh Arctic conditions. In comparison with other aphid species [26, 42, 43, 45], the epithelial cells of vasa deferentia and accessory glands of A. svalbardicum show morphology that is consistent with high secretory activity. These cells are filled with numerous cisternae of rough endoplasmic reticulum, especially in non-copulating individuals. Clearly, it seems to be an adaptation for optimal use of the short breeding period of the Arctic aphids, which is about half the length (30–40 days, from late July to the end of August) compared with their temperate counterparts (60–70 days, from mid September to the end of November). Another interesting feature of the reproductive system, not yet reported in males of any aphid species studied so far, is accumulations of heterogenous secretion in the lumen of the greatly enlarged accessory glands. Ultrastructural analysis of this solid secretion, observed mostly in males that have not copulated, revealed that it is composed of irregularly organized bunches of spicule-like material of high electron density embedded in fine and coarse granular material. Histochemical analysis showed that this material is slightly enriched in proteins. Generally, the secretion of the male accessory glands of insects contains components (mostly proteins and polysaccharides), which promote sperm maturation and nutrition or affect the females’ copulating behavior and physiology [48–50]. Ultrastructural analysis of the reproductive system of copulating pairs of A. svalbardicum suggested that during copulation this material might be transported from the accessory glands of the male to its ejaculatory duct, where it is mixed with the ejaculate, and then is transferred to the spermatheca of the oviparous female (Figure 6). The accessory glands of the males collected alone (i.e., not copulating) were filled with this material. In other insects, e.g., Drosophila melanogaster Meigen, the lumen of male accessory glands was also filled with various filamentous structures, signifying the secretory proteins, which then were observed in mating females [50, 51]. Whether the newly discovered spicule-like material of high electron density produced by the male accessory glands of A. svalbardicum is an additional adaptive feature to the Arctic conditions or influence the behavior and physiology of oviparous females remains a subject of speculation and requires further study, also in other Arctic aphid species.
It should be emphasized that at the end of the growing season, the host plant D. octopetala consists only of vegetative elements in the form of leaves, growing as a dense mat through secondary branching, often covering up to a square meter or more, where it is difficult for males to find oviparous females.
The combination of a cold-hardy overwintering egg and a “risk-free” summer life cycle that also includes some flexibility (i.e., appearance of additional viviparous females generation) with brief synchronized reproduction suggests that A. svalbardicum will be one of the “winners” in a scenario of continued climate warming [24]. A field experiment showed that in controlled conditions, increase of the daily temperature by 1–2°C over the entire summer season at Ny-Ålesund led to a significant increase in the end of summer egg density, from around 500 eggs per square meter in areas without any warming to over 5700 eggs per square meter in controlled patches of D. octopetala [12, 13]. Therefore, despite the conservative structure of the reproductive system of the sexual generation, it should also be characterized by considerable flexibility in response to changing climatic conditions, even within a single growing season.
Conflict of interest
The authors have declared that no conflict of interest exists.
Authors’ Contributions
K.W. has received funding for this study, collected the insect material in the field, conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, and approved the final draft.
D.C. has received funding for this study, collected the insect material in the field, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, and approved the final draft.
Ł.J. analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
P.Ś. conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, and approved the final draft.
Acknowledgments
Our particular thanks to Hannele Savela, Coordinator INTERACT Transnational Access, and Christina A. Pedersen, Manager of the Sverdrup Research Station, Norwegian Polar Institute, Ny-Ålesund. K.W. and D.C. are very grateful to all the staff of Sverdrup Research Station, Norwegian Polar Institute, Ny-Ålesund, for help and hospitality during the field study. The authors are greatly indebted to Danuta Urbańska, Łukasz Chajec, and Mariusz Kanturski for their skilled technical assistance. The authors thank Richard Ashcroft for the linguistic improvement of the manuscript. The authors are also grateful to the Editors and to the four anonymous Reviewers for all valuable comments during the review process.
†Grant support: This study has received funding from the European Union’s Horizon 2020 project INTERACT under Grant Agreement No. 730938 and the National Science Centre, Poland, Grant No. 2015/19/B/NZ9/01265.