Abstract
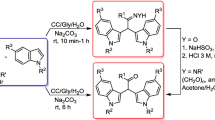
The search for innovative and green reaction media is a prominent topic in the recent research in chemistry. Deep Eutectic Solvents (DESs) are currently gaining relevance in this field thanks to their unique properties in terms of their “greenness” as well as in terms of their catalytic properties. In this work we developed an efficient protocol for the conjugate aza-Michael addition of amines to 2-vinylpyridine using 14 differently structured acid DESs as both reaction media and catalysts, so preventing the use of other acid additives. The results are influenced by the acidity of the components of the DESs, showing the best results with weak acids in the DESs components, with isolated yields up to 86%. The aza-Michael reaction is a widely used synthetic route for the C–N bond formation in organic synthesis. In particular, the addition of amines to 2-vinylpyridine is used for the realization of pharmaceutically relevant compounds with anticancer, antiarrhythmic, and analgesic properties. Comparing the results with the ones observed in literature for the same reaction, this procedure revealed to be a convenient and efficient method for this relevant transformation.
Graphic abstract



Similar content being viewed by others
References
Rehman Z (2001) Bleed control solvents for pigmented and dye-based inks. US patent 6,187,086, 2001; (2000) Chem Abstr 133:194788
Petrelli G, Siepi G, Miligi L, Vineis P (1993) Scand J Work Environ Health 19:63
Freitag W, Stoye D (2008) Paints, coatings and solvents. Wiley, Hoboken
Pace DM, Elliott A (1962) Cancer Res 22:107
Kimura ET, Ebert DM, Dodge PW (1971) Toxicol Appl Pharmacol 19:699
Ali I, Asim M, Khan TA (2012) J Environ Manage 113:170
Aksu Z (2005) Process Biochem 40:997
Li Z, Smith KH, Stevens GW (2016) Chin J Chem Eng 24:215
Spear SK, Griffin ST, Granger KS, Huddleston JG, Rogers RD (2007) Green Chem 9:1008
Meindersma GW, Podt AJG, Meseguer MG, de Haan AB (2005) In: Ionic liquids IIIB: fundamentals, progress, challenges, and opportunities. ACS Publications, p 57
DeSimone JM (2002) Science (80-) 297:799
Germani R, Orlandini M, Tiecco M, Del Giacco T (2017) J Mol Liq 240:233
Paiva A, Craveiro R, Aroso I, M. Martins, Reis RL, Duarte ARC (2014) ACS Sustain Chem Eng 2:1063
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) J Am Chem Soc 126:9142
Araujo CF, Coutinho JAP, Nolasco MM, Parker SF, Ribeiro-Claro PJA, Rudić S, Soares BIG, Vaz PD (2017) Phys Chem Chem Phys 19:17998
Wu S-H, Caparanga AR, Leron RB, Li M-H (2012) Thermochim Acta 544:1
Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH (2013) Anal Chim Acta 766:61
Choi YH, van Spronsen J, Dai Y, Verberne M, Hollmann F, Arends GJ, Witkamp IWCE, Verpoorte R (2011) Plant Physiol 156:1701
Hayyan M, Hashim MA, Hayyan A, Al-Saadi MA, AlNashef IM, Mirghani MES, Saheed OK (2013) Chemosphere 90:2193
Palomba T, Ciancaleoni G, Del Giacco T, Germani R, Ianni F, Tiecco M (2018) J Mol Liq 262:285
Tiecco M, Cappellini F, Nicoletti F, Del Giacco T, Germani R, Di Profio P (2019) J Mol Liq 281:423
Ñíguez DR, Guillena G, Alonso DA (2017) ACS Sustain Chem Eng 5:10649
Alonso DA, Baeza A, Chinchilla R, Guillena G, Pastor IM, Ramón DJ (2016) Eur J Org Chem 2016:612
Punzi A, Coppi DI, Matera S, Capozzi MAM, Operamolla A, Ragni R, Babudri F, Farinola GM (2017) Org Lett 19:4754
Vidal C, García-Álvarez J, Hernán-Gómez A, Kennedy AR, Hevia E (2014) Angew Chem 126:6079
Ma C, Laaksonen A, Liu C, Lu X, Ji X (2018) Chem Soc Rev 47:8685
Gabriele F, Chiarini M, Germani R, Tiecco M, Spreti N (2019) J Mol Liq 291:111301
Brinchi L, Germani R, Braccalenti E, Spreti N, Tiecco M, Savelli G (2010) J Colloid Interface Sci 348:137
Di Crescenzo A, Tiecco M, Zappacosta R, Boncompagni S, Di Profio P, Ettorre V, Fontana A, Germani R, Siani G (2018) J Mol Liq 268:371
Curti F, Tiecco M, Pirovano V, Germani R, Caselli A, Rossi E, Abbiati G (2019) Eur J Org Chem 2019:1904
Cannalire R, Tiecco M, Cecchetti V, Germani R, Manfroni G (2018) Eur J Org Chem 2018:2977
Tiecco M, Germani R, Cardellini F (2016) RSC Adv 6:43740
Lonnon DG, Craig DC, Colbran SB (2006) Dalton Trans 3785
Almansa-Rosales C, Garcia-Lopez M, Garriga-Sanahuja L, Llorente-Fernandez AV (2019) Preparation of 1-methylpyrazole-piperazine compounds having multimodal activity against pain. U.S. patent 10,189,828, Jan 29, 2019; (2016) Chem Abstr 165:115117
Oinuma H, Yamanaka M, Miyake K, Hoshiko T, Minami N, Shoji T, Nomoto K (1992) Preparation of [(sulfonylamino)benzoyl]piperidines as antiarrhythmic agents. U.S. patent 5,118,689, Jun 2, 1992; (1988) Chem Abstr 108:167305
Akhtar J, Khan AA, Ali Z, Haider R, Yar MS (2017) Eur J Med Chem 125:143
Taki M, Teramae S, Nagatomo S, Tachi Y, Kitagawa T, Itoh S, Fukuzumi S (2002) J Am Chem Soc 124:6367
Ross WF, Walters DR, Robins DJ (2004) Pest Manag Sci 60:143
Cardellini F, Tiecco M, Germani R, Cardinali G, Corte L, Roscini L, Spreti N (2014) RSC Adv 4:55990
Cardellini F, Germani R, Cardinali G, Corte L, Roscini L, Spreti N, Tiecco M (2015) RSC Adv 5:31772
De Santi V, Cardellini F, Brinchi L, Germani R (2012) Tetrahedron Lett 53:5151
Abbott AP, Barron JC, Ryder KS, Wilson D (2007) Chem Eur J 13:6495
Nejrotti S, Iannicelli M, Jamil SS, Arnodo D, Blangetti M, Prandi C (2020) Green Chem 22:110
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Chem Commun 70
Weast RC (1971) Handbook of Chemistry and Physics. OH, Chem Rubber Co, Cleveland, p C528
Kirk RE, Othmer DF, Mann CA (1949) Encyclopedia of Chemical Technology, vol II. J Phys Chem 53:591
Romary JK, Barger JD, Bunds JE (1968) Inorg Chem 7:1142
Kumari T, Chauhan R, Sharma N, Kaur K, Krishnamurthy A, Pandey P, Aggarwal S (2016) J Undergrad Res Innov 2:203
Rolff M, Hamann JN, Tuczek F (2011) Angew Chem Int Ed 50:6924
Ghasemi MH, Kowsari E, Shafiee A (2016) Tetrahedron Lett 57:1150
Zhang SF, Qian QL, Chen Y, Yuan GQ (2004) Chem J Chin Univ 25:2019
Zhu Z, Chen H, Li S, Yang X, Bittner E, Cai C (2017) Catal Sci Technol 7:2474
Beller M, Trauthwein H, Eichberger M, Breindl C, Müller TE (1999) Eur J Inorg Chem 1999:1121
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ballarotto, M., Cappellini, F., Maestri, R. et al. Exploring the acidic catalytic role of differently structured deep eutectic solvents in the aza-Michael addition of amines to 2-vinylpiridine. Monatsh Chem 151, 1387–1394 (2020). https://doi.org/10.1007/s00706-020-02660-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02660-z