Abstract
Purpose
To keep and increase spermatogonial stem cell number (SSC) is the only available option for pediatric cancer survivors to maintain fertility. Leptin is secreted by the epididymal white adipose tissue and has receptors on stem/progenitor spermatogonia. The purpose of this study is to demonstrate dose- and time-dependent proliferative effect of leptin on stem/progenitor spermatogonia cultures from prepubertal mice testes.
Methods
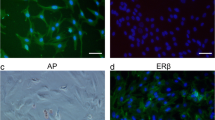
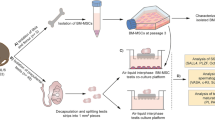
CD90.2 (+) stem/progenitor spermatogonia were isolated from the C57BL/6 mouse testis on postnatal day 6 and placed in culture. The proliferative effect of leptin supplementation was assessed by colony formation (diameter and number), WST proliferation assays, and xCELLigence real-time cell analysis (RTCA) on days 3, 5, and 7 of culture. Expressions of p-ERK1/2, p-STAT3, total STAT3, and p-SHP2 levels were determined by western blot analysis.
Results
Leptin supplementation of 100 ng/ml increased the diameter (p = 0.001) and number (p = 0.01) of colonies in stem/progenitor spermatogonial cultures and caused higher proliferation by WST-1 (p = 0.009) compared with the control on day 7. The EC50 was calculated as 114 ng/ml for leptin by RTCA. Proliferative dose of leptin induced increased expression of p-ERK1/2 (p = 0.009) and p-STAT3 (p = 0.023) on stem/progenitor spermatogonia when compared with the untreated group.
Conclusion
The results indicated that leptin supplementation exhibited a dose- and time-dependent proliferative effect on stem/progenitor spermatogonia that was associated with increased expression of ERK1/2 and STAT3 pathways while maintaining their undifferentiated state. This output presents a new agent that may help to expand the stem/progenitor spermatogonia pool from the neonatal testis in order to autotransplant after cancer treatment.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gholami M, Hemadi M, Saki G, Zendedel A, Khodadadi A, Mohammadi-Asl J. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability? J Assist Reprod Genet. 2013;30(10):1271–7.
van Casteren NJ, van der Linden GH, Hakvoort-Cammel FG, Hahlen K, Dohle GR, van den Heuvel-Eibrink MM. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer. 2009;52(1):108–12.
Mei XX, Wang J, Wu J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J Androl. 2015;17(3):347–54.
von Kopylow K, Schulze W, Salzbrunn A, Spiess AN. Isolation and gene expression analysis of single potential human spermatogonial stem cells. Mol Hum Reprod. 2016;22(4):229–39.
Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23(11):2402–14.
Galuppo AG. Spermatogonial stem cells as a therapeutic alternative for fertility preservation of prepubertal boys. Einstein (Sao Paulo). 2015;13(4):637–9.
Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149(4):R159–67.
Potter SJ, DeFalco T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction. 2017;153(4):R151–R62.
Kose S, Yersal N, Onen S, Korkusuz P. Comparison of hematopoietic and spermatogonial stem cell niches from the regenerative medicine aspect. Adv Exp Med Biol. 2018;1107:15–40.
Jalali AS. Epididymal white adipose tissue: endocrine backbone of spermatogonial stem cells maintenance. J Stem Cell Biol Transplant. 2017;01(03):17
Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology. 2010;151(12):5669–79.
Almabhouh FA, Osman K, Siti Fatimah I, Sergey G, Gnanou J, Singh HJ. Effects of leptin on sperm count and morphology in Sprague-Dawley rats and their reversibility following a 6-week recovery period. Andrologia. 2015;47(7):751–8.
Hart RA, Dobos RC, Agnew LL, Smart NA, McFarlane JR. Leptin pharmacokinetics in male mice. Endocr Connect. 2017;6(1):20–6.
Zhang J, Gong M. Review of the role of leptin in the regulation of male reproductive function. Andrologia. 2018;50(4)
Herrid M, O'Shea T, McFarlane JR. Ontogeny of leptin and its receptor expression in mouse testis during the postnatal period. Mol Reprod Dev. 2008;75(5):874–80.
Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol. 2013;13(1):1–14.
Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia. 2007;39(1):22–7.
Roumaud P, Martin LJ. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm Mol Biol Clin Invest. 2015;24(1):25–45.
Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, et al. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27(2):302–10.
Martins FF, Aguila MB, Mandarim-de-Lacerda CA. Impaired steroidogenesis in the testis of leptin-deficient mice (ob/ob -/-). Acta Histochem. 2017;119(5):508–15.
Hoffmann A, Manjowk GM, Wagner IV, Kloting N, Ebert T, Jessnitzer B, et al. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology. 2016;157(6):2461–8.
Schoeller EL, Chi M, Drury A, Bertschinger A, Esakky P, Moley KH. Leptin monotherapy rescues spermatogenesis in male Akita type 1 diabetic mice. Endocrinology. 2014;155(8):2781–6.
Esmaili-Nejad MR, Babaei H, Kheirandish R. The effects of long-term leptin administration on morphometrical changes of mice testicular tissue. Iran J Basic Med Sci. 2015;18(12):1176–82.
Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, et al. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272(20):12897–900.
Wagoner B, Hausman DB, Harris RB. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Phys Regul Integr Comp Phys. 2006;290(6):R1557–64.
Yu T, Luo G, Zhang L, Wu J, Zhang H, Yang G. Leptin promotes proliferation and inhibits differentiation in porcine skeletal myoblasts. Biosci Biotechnol Biochem. 2008;72(1):13–21.
Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, Wicha MS, et al. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prev Res (Phila). 2015;8(12):1174–83.
Taskin AC, Kocabay A, Ebrahimi A, Karahuseyinoglu S, Sahin GN, Ozcimen B, et al. Leptin treatment of in vitro cultured embryos increases outgrowth rate of inner cell mass during embryonic stem cell derivation. In Vitro Cell Dev Biol Anim. 2019;55(7):473–81.
Artwohl M, Roden M, Holzenbein T, Freudenthaler A, Waldhausl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26(4):577–80.
Goren I, Pfeilschifter J, Frank S. Determination of leptin signaling pathways in human and murine keratinocytes. Biochem Biophys Res Commun. 2003;303(4):1080–5.
Huang F, Xiong X, Wang H, You S, Zeng H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappa B. Acta Biochim Biophys Sin Shanghai. 2010;42(5):325–31.
Tsai YC, Lee YM, Hsu CH, Leu SY, Chiang HY, Yen MH, et al. The effect of ferulic acid ethyl ester on leptin-induced proliferation and migration of aortic smooth muscle cells. Exp Mol Med. 2015;47:e180.
Li L, Gao Y, Zhang LL, He DL. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther. 2008;7(11):1787–92.
Yoon KW, Park SY, Kim JY, Lee SM, Park CH, Cho SB, et al. Leptin-induced adhesion and invasion in colorectal cancer cell lines. Oncol Rep. 2014;31(6):2493–8.
Oliver P, Pico C, Palou A. Ontogenesis of leptin expression in different adipose tissue depots in the rat. Pflugers Arch. 2001;442(3):383–90.
Hansel W. The essentiality of the epididymal fat pad for spermatogenesis. Endocrinology. 2010;151(12):5565–7.
Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13(2):629–40.
Magarinos MP, Sanchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007;76(2):203–10.
Fontoura P, Mello MD, Gallo-Sa P, Erthal-Martins MC, Cardoso MC, Ramos C. Leptin improves sperm cryopreservation via antioxidant defense. J Reprod Infertil. 2017;18(1):172–8.
Niu Z, Goodyear SM, Avarbock MR, Brinster RL. Chemokine (C-X-C) Ligand 12 facilitates trafficking of donor spermatogonial stem cells. Stem Cells Int. 2016;2016:5796305.
Azizi H, Skutella T, Shahverdi A. Generation of mouse spermatogonial stem-cell-colonies in a non-adherent culture. Cell J. 2017;19(2):238–49.
Wang P, Suo LJ, Wang YF, Shang H, Li GX, Hu JH, et al. Effects of GDNF and LIF on mouse spermatogonial stem cells proliferation in vitro. Cytotechnology. 2014;66(2):309–16.
Wang P, Zheng Y, Li Y, Shang H, Li GX, Hu JH, et al. Effects of testicular interstitial fluid on the proliferation of the mouse spermatogonial stem cells in vitro. Zygote. 2014;22(3):395–403.
Mohamadi SM, Movahedin M, Koruji SM, Jafarabadi MA, Makoolati Z. Comparison of colony formation in adult mouse spermatogonial stem cells developed in Sertoli and STO coculture systems. Andrologia. 2012;44(Suppl 1):431–7.
Navid S, Rastegar T, Baazm M, Alizadeh R, Talebi A, Gholami K, et al. In vitro effects of melatonin on colonization of neonate mouse spermatogonial stem cells. Syst Biol Reprod Med. 2017;63(6):370–81.
Bai Y, Feng M, Liu S, Wei H, Li L, Zhang X, et al. Differential gene expression in mouse spermatogonial stem cells and embryonic stem cells. Int J Mol Med. 2016;38(2):423–32.
Corotchi MC, Popa MA, Simionescu M. Testosterone stimulates proliferation and preserves stemness of human adult mesenchymal stem cells and endothelial progenitor cells. Romanian J Morphol Embryol. 2016;57(1):75–80.
Mendi A, Yagci BG, Kiziloglu M, Sarac N, Yilmaz D, Ugur A, et al. Effects of Syzygium aromaticum, Cinnamomum zeylanicum, and Salvia triloba extracts on proliferation and differentiation of dental pulp stem cells. J Appl Oral Sci. 2017;25(5):515–22.
Pakdemirli A, Toksoz F, Karadag A, Misirlioglu HK, Baspinar Y, Ellidokuz H, et al. Role of mesenchymal stem cell-derived soluble factors and folic acid in wound healing. Turk J Med Sci. 2019;49(3):914–921
Kubota H, Brinster RL. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008;86:59–84.
Oatley JM, Kaucher AV, Avarbock MR, Brinster RL. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod. 2010;83(3):427–33.
Shakeri M, Kohram H, Shahverdi A, Shahneh AZ, Tavakolifar F, Pirouz M, et al. Behavior of mouse spermatogonial stem-like cells on an electrospun nanofibrillar matrix. J Assist Reprod Genet. 2013;30(3):325–32.
Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cell Dev Biol Anim. 2009;45(5-6):281–9.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97(15):8346–51.
Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100(11):6487–92.
Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71(3):722–31.
Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73(5):1011–6.
Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–9.
El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141(7):2624–30.
Banks WA, McLay RN, Kastin AJ, Sarmiento U, Scully S. Passage of leptin across the blood-testis barrier. Am J Phys. 1999;276(6):E1099–104.
Wang X, Zhang X, Hu L, Li H. Exogenous leptin affects sperm parameters and impairs blood testis barrier integrity in adult male mice. Reprod Biol Endocrinol. 2018;16(1):55.
Haron MN, D'Souza UJ, Jaafar H, Zakaria R, Singh HJ. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertil Steril. 2010;93(1):322–4.
Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20.
Moreira BP, Monteiro MP, Sousa M, Oliveira PF, Alves MG. Insights into leptin signaling and male reproductive health: the missing link between overweight and subfertility? Biochem J. 2018;475(22):3535–60.
Puri P, Phillips BT, Suzuki H, Orwig KE, Rajkovic A, Lapinski PE, et al. The transition from stem cell to progenitor spermatogonia and male fertility requires the SHP2 protein tyrosine phosphatase. Stem Cells. 2014;32(3):741–53.
Puri P, Walker WH. The regulation of male fertility by the PTPN11 tyrosine phosphatase. Semin Cell Dev Biol. 2016;59:27–34.
Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19(9):2075–83.
Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12(13):2048–60.
Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96(6):2846–51.
Zhang Y, Wang D, Xu J, Wang Y, Ma F, Li Z, et al. Stat3 activation is critical for pluripotency maintenance. J Cell Physiol. 2019;234(2):1044–51.
Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–5.
Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294(5551):2546–9.
Liu Y, Lv L, Xiao W, Gong C, Yin J, Wang D, et al. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. J Huazhong Univ Sci Technolog Med Sci. 2011;31(3):365–70.
Funding
The Research Coordination Unit of Hacettepe University funded this work (THD-2017-13430). Professor Kyle E Orwig was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD092084.
Author information
Authors and Affiliations
Contributions
Nilgun Yersal generated the hypothesis, established the rationale and designed the study together with her mentor Petek Korkusuz, and wrote the manuscript. Nilgun Yersal performed the experimental study, cultured the stem/progenitor spermatogonia, and performed histological examination of the testes. Utku Horzum analyzed and interpreted the flow cytometry and western blotting data. Sevil Kose supervised the MACS isolation and interpreted the xCELLigence data. Sinan Ozkavukcu contributed to the analysis of the cell data. Dr Kyle E Orwig, as the co-advisor of Nilgun Yersal, supervised the Ob receptor immune labeling; Petek Korkusuz and Kyle E Orwig edited the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Hacettepe University Animal Experimentations Local Ethics Board (#2016/59/1) approved the use of animal material.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yersal, N., Köse, S., Horzum, U. et al. Leptin promotes proliferation of neonatal mouse stem/progenitor spermatogonia. J Assist Reprod Genet 37, 2825–2838 (2020). https://doi.org/10.1007/s10815-020-01929-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01929-w