Abstract
Steppes and xerothermic grasslands are hotspots of biodiversity, but are threatened by habitat destruction and fragmentation. The heath bush-cricket, Gampsocleis glabra, is considered to be a specialist of xerothermic habitats and appears in national red lists as a threatened species in several European countries. The goal of the current research was to determine the habitat requirements of G. glabra in an isolated habitat patch in Poland, at the northern edge of its range. By comparing the composition of plant species and vegetation architecture of vacant and occupied sites in the summers of 2018 and 2019, it was found that this population of G. glabra still maintained a strict specialisation for the xerothermofilous Festuco-Brometea plant community. On the contrary to previous studies, however, Stipa-type grasses were not essential for the occurrence of the species and the majority of occupied areas were based on the plant Brachypodium pinnatum. The physiognomy of plant communities was crucial for the abundance of stridulating males, which showed a preference for dense grasses at 10 cm high. The habitat characteristics of patches occupied by males and females did not differ significantly. The study of habitat requirements of this endangered Orthoptera species in an isolated habitat patch could serve as a prelude to the restoration of similar locations before it becomes extinct. This study may also underpin the development of a global conservation strategy for G. glabra.
Similar content being viewed by others
Introduction
Steppes and xerothermic grasslands are among the most valuable and the most endangered habitats in Europe (Török et al. 2016). Such habitats were widely distributed throughout Europe during glacial periods (Willis and van Andel 2004; Markova et al. 2009; Kajtoch et al. 2016) and were limited to eastern and south-eastern regions during interglacials (Fekete et al. 2014; Pokorný et al. 2015; Kajtoch et al. 2016). Today’s xerothermophilous populations are probably remnants of much larger populations of species that were widely distributed during glaciations (Kajtoch et al. 2016). Xerothermic habitats from the class Festuco-Brometea are hotspots for plant and insect diversity (Kajtoch 2011; Wilson et al. 2012). Findings of studies conducted on steppes and xerothermic grasslands can help develop appropriate conservation strategies but are still insufficient (Valko et al. 2016). Such habitats can be an excellent place to complement knowledge gaps concerning the extinction of rare species (Steffan-Dewenter and Tscharntke 2002).
It is known that rare species inhabiting steppes and xerothermic grasslands depend on habitat quality (Thomas et al. 2001; Poniatowski et al. 2018). Plant diversity of habitats has a positive effect on species richness, regardless of the patch size and mobility of organisms (Marini et al. 2010; Kajtoch et al. 2014). Plant species composition and diversity are important for grasshoppers, bushcrickets, crickets and relatives (Gardiner 2010; Lengyel et al. 2016; Gardiner 2018). Orthoptera species may be, however, negatively affected by habitat fragmentation (Andrén 1994; Krauss et al. 2010; Poniatowski et al. 2016) and low habitat patch connectivity (Poniatowski et al. 2016). It is known that isolated patches are more exposed to species decline than fragmented, but connected, areas (Collinge 2000). In the case of grassland specialists, the area of the patch and habitat quality are of key importance, while patch connectivity is less important (Poniatowski et al. 2018). Therefore, the protection of plant diversity of patchy steppes and xerothermic grasslands seems to be crucial for the survival of rare insect species (including Orthoptera) associated with these habitats.
Some research indicates that the occurrence of xerothermophilous insects, like Decticus verrucivorus and Platycleis albopunctata, depends on the presence of a habitat mosaic. Both species used different places e.g. to search for food and for oviposition (Schirmel et al. 2010). However, these species are mobile and the presence of several isolated patches of steppe was sufficient for them to move. The habitat quality of a single patch tends to be more important for flightless and sedentary Orthoptera species with low dispersal ability (Poniatowski and Fartmann 2010). For example, the degree of patch occupancy increased with patch size in case of Metrioptera brachyptera in calcareous grasslands. In this case, patch occupancy was independent of the degree of isolation, which is not surprising because of the insects’ low dispersal ability. The population density was also determined by the habitat, as it generally increased with vegetation height (Poniatowski and Fartmann 2010).
To develop a conservation strategy, knowledge of habitat factors favourable and unfavourable for the presence of rare insects in the patch is needed. The greatest threat of extinction concerns species which are highly specialized to the specific environment and also those living in isolated patches. An example of such a habitat specialist is the heath bush-cricket Gampsocleis glabra, which lives in xerothermic habitats (Schouten et al. 2007), and has been associated with steppes containing grasses of the Stipa genus (Liana 1976; Liana 2007; Stahi and Derjanschi 2011). We need to know the responses of rare species to habitat quality after isolation (Pilskog et al. 2016) to protect them from extinction. The key is to understand the importance of the composition of plant species and vegetation architecture (Krausz et al. 1995).
This study aims to determine habitat requirements of G. glabra living in the isolated Polish population. We test the following hypotheses: (1) sites occupied by males and females of G. glabra differ in habitat features from the vacant sites, (2) G. glabra is a specialist of the xerothermic plant community, even in an isolated patch, (3) the presence of Stipa grasses determines the occurrence of G. glabra, as in Poland this species has been assumed to be an indicator of the xerothermic Sisymbrio-Stipetum grasslands (Liana 1976). In addition, the location of non-volatile nymphs were identified in the field to verify the degree of mobility of the species.
Materials and methods
Study species and study site
Gampsocleis glabra has a West-Siberian and European distribution (Ingrisch and Köhler 1998). It figures in the European national red lists as an endangered or critically endangered species in Austria (Berg and Zuna-Kratky 1997), Germany (Maas et al. 2002), Poland (Liana 2007), and Moldova (Stahi and Derjanschi 2011). It has declined in the East-Slovakian lowland (Krištín et al. 2004), and is rare in Slovakia, although lately it has become more frequent in some parts of this country (Krištín et al. 2007, 2011). It is also rare in Romania (Iorgu and Iorgu 2008), and absent in the Transcarpathian Ukraine (Krištín et al. 2011). It reaches the northern edge of its range in Poland (Hochkirch 2014), and it is extinct in the neighbouring Czech Republic (Holuša 2012). Although its IUCN category is LC (least concern), the number of heath bush-crickets in Europe and Asia shows a downward trend (Hochkirch 2014). Research on the isolated Polish population is particularly urgent.
Adult G. glabra can be found from mid-June to the end of September, with a peak of abundance in July (Krištín et al. 2007). Males stridulate from perches in the vegetation during the day to attract females and tend to be regularly spaced in suitable habitat (Latimer 1980). Females use their long ovipositor to lay eggs in the soil (Vahed 1994). The eggs can have both an initial diapause and an embryonic diapause and can pass two or even three winters in the egg stage before they hatch in the spring (Hartley 1990). The species feeds on insects, grasses and grass seeds (Vahed 1994).
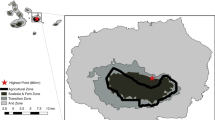
The study was carried out on xerothermic grasslands in south-eastern Poland, in the Nida Basin, which is a part of the Małopolska Upland, located north of Kraków (Fig. 1). Steppe grasslands in the Nida Basin are isolated from other similar habitats in Poland. Moreover, the Polish Uplands, on which those grasslands are located, are isolated from similar habitats in western and southern Europe, linked only with the Podolian Uplands in Ukraine (Kajtoch et al. 2016). Xerothermic habitats in the Nida Basin come from natural steppes but are affected by land use, especially agriculture (Towpasz 2011; Kajtoch et al. 2016). In the 1970s, there were at least five locations of G. glabra in the Małopolska Upland (Liana 1976), of which the only one within the Nida Basin has survived until the present. This location is a xerothermic compact patch with an area of about 15 ha on a small hill surrounded by an agricultural landscape (Fig. 2a, b).
a Hilly patch with steppe habitat, in which G. glabra lives, with a view of the surrounding agricultural landscape; b the periphery of the patch, along which one of the marginal transects for the acoustic detection of insects has been designated; c male G. glabra during stridulation; d a nymph of G. glabra on the studied isolated patch. The photo shows an immature male (photographs by: E. Grzędzicka)
Field protocol
The study was conducted from June to August 2018 and from May to August 2019. In June 2018, three transects were marked out, leading through the centre of the patch and along its two long edges. The two marginal transects were at a distance of 2–4 m from the border, depending on how it was possible to move on the hilly terrain (Fig. 2b). Three transects ran through the wider half of habitat, and in the second (narrower) half, only one marginal transect was continued. The presence of G. glabra was checked by acoustic identification of stridulating males based on the methodology of Krištín et al. (2004, 2007, 2011). Acoustic stridulation of Orthoptera males is useful in species classification and in assessing population density (Fischer et al. 1997).
In 2018, transects were visited between 9 and 15 a.m. in the third week of June, in the third week of July and in the third week of August by one person (EG) under favourable weather conditions. The research was not performed on cold days, because temperature affects the acoustic behaviours and song traits of Orthoptera (von Helversen 1972; Pasinelli et al. 2013). Acoustic insect searches on each transect and each field inspection were limited to 2 h (Krištín et al. 2004). In most cases, stridulating males were easily noticeable. Females, in turn, were very secretive and hidden in the vegetation. The males usually sang after climbing a selected strong blade of grass or other plant (Fig. 2c). To ensure the correct species identification, the stridulation of males was recorded with a digital recorder Song Meter SM4 (Wildlife Acoustics, Inc., Maynard, Massachusetts, USA) located about 1 m from the insect. Insects were not captured, so vegetation remained unchanged, and animal stress was minimal.
In May and June 2019, the presence of nymphs was checked (Fig. 2d). In the third week of July (peak of the heath bush-cricket) in 2019, stridulating males were located using transects designated in 2018. At 1-day inspections in June, July and August, females were found and the habitat around them was immediately described (see below).
Habitat characteristics
In 2018, 28 sites were marked in the form of 1 × 1 m squares, centred upon locations of calling male G. glabra. The number 28 is a random number of singing males found at inspections in June or July. A Song Meter SM4 was used to record the geographical position of the point located about 1 m from the singing male and half a meter from the edge of a 1 × 1 m square defined around the insect. Thus, the site could be found during other inspections. Squares around stridulating males on the researched patch were located at least 3 m from its edge. To compare the occupied and unoccupied habitats, 25 squares with the same areas (1 × 1 m) without the researched species were designated. Squares without G. glabra were chosen at least 3 m from the occupied places, but no more than 10 m from the nearest occupied space, and at least 3 m from the habitat edge. These assumptions ensure that the comparison of occupied and unoccupied positions concerned habitat parameters without an edge effect. Both types of sites were placed in a mosaic in the same parts of the study area.
In 2018, the habitat within squares was also assessed twice per site in June and July to capture the full species richness of plants. Each square was characterized by one phytosociological relevé with the list of plant species and the relative coverage of plant species estimated in squares based on the Braun-Blanquet scale. Plant identification was based on Medwecka-Kornaś and Kornaś (1972), Łuszczyńska (1998), Mirek et al. (2002), Kostuch and Misztal (2006) and Misztal et al. (2015). Plant species were classified to meadow or xerothermic plant communities (Matuszkiewicz 2005; Suppl. Table S2). The following habitat indicators were recorded on each square: number of all plant species, number of xerothermic species, number of meadow species, cover of each of these two types of plants, cumulative (summarised) coverage of all plants expressed in the Braun-Blanquet scale, cumulative coverage of xerothermic and meadow species separately in the Braun-Blanquet scale, their percentages in the cumulative coverage of plants, cover of the Stipa capillata and Brachypodium pinnatum in the Braun-Blanquet scale. Also, a total cover of vegetation at 10 cm above the ground and at 40 cm above the ground was noted. In cases of varying values, the higher one was chosen from the two recorded on two field inspections.
In June and July 2019, 53 squares used for habitat descriptions in the previous year were used again. In cases of sites occupied by males of G. glabra in 2018, the closest stridulating males in the second year of the study were found within those 1 × 1 m squares or within a maximum of 1.5 m from the center of the square. Thus, it was assumed that male sites from 2018 were occupied also in 2019. All phytosociological relevés from 2018 were updated on each square in 2019. Sites that were not occupied by G. glabra in 2018 were also unoccupied in 2019. In July 2019, 15 females were found at a distance of 1–3 m from the nearest stridulating male and one relevé with an area of 1 × 1 m around each female was prepared immediately. Squares describing the habitat around females did not fully overlap with male site habitats nor with squares where the studied insect was searched and not found.
Statistical analyses
The distribution of data was verified using the Shapiro-Wilk test based on the results of 53 phytosociological relevés. Normally distributed parameters were compared using the Student t-test for unpaired comparison, while non-parametric Mann-Whitney U test was used for other habitat features, in both cases using JMP 8 software.
To check which habitat features determined the presence of G. glabra, a logistic regression model with a dependent variable having binomial distribution and logit link function was performed using data collected in 2018. The following habitat features per square were used as factors in the model: number of plant species, number of xerothermic plant species, number of meadow plant species, plant cover, summarized cover of xerothermic plants, summarized cover of meadow plants, cover of S. capillata, cover of B. pinnatum, percent cover of vegetation at 10 cm above the ground and cover at 40 cm. Because habitat parameters were correlated with each other, the strength of multicollinearity was detected using the GGally package (Schloerke et al. 2018) and the location of multicollinearity with Spearman correlation coefficient in corpcor package (Schafer et al. 2017). Then the mctest package (Imdadullah et al. 2016) was used to test if the model suffered from multicollinearity. The intercorrelated factors were removed from the model. However, to check to what extent the rejected factors explained the occurrence or absence of G. glabra, principal component analysis PCA was performed using factoextra (Kassambara and Mundt 2019), FactoMineR (Le et al. 2008) and ggplot2 (Wickham 2016) packages.
To compare the qualitative and quantitative composition of G. glabra male and female habitat and uninhabited sites, PCA based on particular plant species covers was conducted. In this analysis, habitat data from 53 males’ squares and female habitat data collected in 2019 were used. Among the list of plant species, only those present in at least 5 phytosociological relevés were selected. To check which habitat features could differentiate habitat occupancy by different sexes and whether these differences were statistically significant, a linear regression model was calculated. Factors in the model were as follows: coverage of the square with common meadow species of grass Poa trivialis, coverage with meadow species of grass Arrhenatherum elatius, cumulative coverage of meadow plants, coverage of B. pinnatum, coverage of S. capillata and cumulative coverage of xerothermic species of plants. All the above models, analyses and graphs were conducted in R 3.6.2 (R Core Team 2019).
Results were treated significant if P < 0.05.
Results
A total of 112 plant species were recorded on the 28 occupied and 25 unoccupied sites (Suppl. Table S2). 49 plant species were xerothermic, mostly from the Festuco-Brometea class, and 63 were typical meadow plant species from the Molinio-Arrhenatheretea class. The species richness of plants based on their cumulative coverage was on average 20% higher in sites occupied by G. glabra. This resulted from a higher number and coverage of xerothermic plant species (Table 1). In contrast, unoccupied sites were characterized by a higher number and coverage of meadow plant species (Table 1). Generally, sites occupied by males of G. glabra had higher xerothermic coverage than unoccupied squares.
Grasses of the Stipa genus did not grow in sites without stridulating males (Table 1). On the other hand, S. capillata grew only in 36% of the research areas inhabited by G. glabra. The tor-grass B. pinnatum, typical for xerothermic grasslands from the Festuco-Brometea class, grew in 93% of patches inhabited by G. glabra and in 16% of the other examined areas. Taking into account the results from all squares, the mean coverage of B. pinnatum from sites occupied by G. glabra was about 95% higher than in unoccupied sites (Table 1). In 21 sites without stridulating males, B. pinnatum was absent, while in the other four ones, its coverage was very low. 90% of squares occupied by G. glabra contained B. pinnatum with coverage of 20–75%. Squares with G. glabra had an average vegetation coverage of 96.7% at 10 cm above the ground (about 10% more than in the case of squares without an insect—Table 1), while at a height of 40 cm, only 35.4%. The plant cover at 40 cm was on average 40% lower than on unoccupied sites (Table 1).
The logistic regression model showed that the number of xerothermic plant species, Brachypodium sp. grass cover and vegetation at 10 cm positively influenced the presence of G. glabra (Table 2; Figs. 3 and 4; Suppl. Fig. S1), while the number of meadow species and meadow cover had the opposite effect (Table 2; Fig. 4; Suppl. Fig. S1). PCA revealed a high degree of interrelatedness among habitat variables (Fig. 4). The two-dimensional PCA plot revealed two groups of interrelated variables. The first two axes explained approximately 81.5% of the total variance. Dim1 (correlated with: total plant coverage, the number of all plant species, xerothermic plants’ cover, number of xerothermic species, Stipa sp. coverage, Brachypodium sp. coverage and coverage of vegetation at 10 cm) had a significant impact on the insect occurrence. Dim2 (correlated with: meadow cover, number of meadow species, vegetation cover at 40 cm) concerned sites not inhabited by G. glabra (Fig. 4).
PCA concerning data from 2019 confirmed the differences in plant communities between occupied and unoccupied squares (Fig. 5), although the comparison based on the coverage of individual plant species explained only 30.3% of variance. The female habitat was only slightly wider than that of males in terms of the share of meadow species (Fig. 5). The habitats occupied by males and females of G. glabra were similar and none of the habitat features determined the presence of only one sex (Suppl. Table S1).
PCA chart showing differences between sites occupied by G. glabra males and females and unoccupied squares. Plants in sites occupied by G. glabra: Astr dan = Astragalus danicus, Phle phle = Phleum phleoides, Car flac = Carex flacca, Fest stri = Fesctuca stricta, Ave prat = Avenastrum pratense, Dian car = Dianthus carthusianorum, Gal vald = Galium valdepilosum, Brom ine = Bromus inermis, Carl aca = Carlina acaulis, Stip cap = Stipa capillata, Ado vern = Adonis vernalis, Camp sib = Campanula sibirica, Brach pin = Brachypodium pinnatum, Gal lino = Galatella linosyris, Mela arv = Melampyrum arvense, Inul ens = Inula ensifolia, Scab och = Scabiosa ochroleuca, Thal min = Thalictrum minus, Scab col = Scabiosa columbaria; plants in unoccupied squares: Arrh ela = Arrhenatherum elatius, Agro stol = Agrostis stolonifera, Holc lan = Holcus lanatus, Poa triv = Poa trivialis, Gal ver = Galium verum, Anth vul = Anthyllis vulneraria, Dact glom = Dactylis glomerata, Lol per = Lolium perenne. Chart is based on plant covers on 53 squares prepared in 2018 but updated in 2019; 15 squares with the female habitat were described in the year 2019
In May and June 2019, nymphs were observed in the central part of the isolated studied patch (Fig. 2d). During 4 days of searching, about 20 nymphs were found.
Discussion
The present study shows that sites chosen by the heath bush-cricket differ significantly from unoccupied squares in a range of habitat characteristics, based on the composition of plant species and vegetation architecture. Thus, even in the patch of habitat isolated from the species’ main range, G. glabra still maintains preferences for steppes and xerothermic grasslands from the Festuco-Brometea class. In contrast, in two neighbouring countries, this species has not been found in thermophilous habitats: in the East-Slovakian lowland (Krištín et al. 2004) and SW Ukraine (Krištín et al. 2011). G. glabra was unexpectedly among the most frequent insects in dunes and xeric sandy stands surrounded by wet grassy depressions located in SE Slovakia (Krištín et al. 2007, 2011), with 54% occurrence frequency (Krištín et al. 2011).
It interesting that in Poland, the relatively small habitat plasticity (strong connection with xerothermic habitat) of G.glabra does not seem to lead to its extinction, because the studied population is known to have occupied the location for over 50 years (Liana 1976). On the other hand, this species in Poland was once considered to be an indicator of the xerothermic Sisymbrio-Stipetum grasslands (Liana 1976). Excluding S. capillata, no other plant species from the Sisymbrio-Stipetum community grew in the 53 relevés in the present study area. This may be the result of habitat degradation and changes in land use. The study area used to be a grazed community, but this was discontinued in early 2000 s. The high grassland typical of the steppes is disappearing in the study area, and thin, xerothermic grasslands with a clumped structure are developing instead (Towpasz and Stachurska-Swakoń 2012). Therefore, it cannot be stated that the Polish heath bush-cricket population does not show any habitat plasticity. It still lives in the location previously described as the one with the largest G. glabra population size among a few in Poland (Liana 1976), despite a decline of some xerothermic plants.
In the PCA analysis, it was shown that Stipa sp. grass is a component of the system that promotes the presence of G. glabra. Despite the higher cover of S. capillata in squares with G. glabra than in others, these grasses were not necessary for the singing males, which is surprising. In 2018, S. capillata grew only in 36% of the research areas inhabited by G. glabra. Interestingly, S. capillata was present also in some of the surveyed positions in Slovakia, even if it was not a dominant species in the area of occurrence of the examined insects (Krištín et al. 2011).
The present study reveals that in Poland, G. glabra occupies sites of specific physiognomy. Squares occupied by the heath bush-cricket were dominated by B. pinnatum, which is a characteristic species of the xerothermic plant community. In this case, the plant community containing B. pinnatum resembles the physiognomy of steppes with tall, rather dense stems, freely moving in the wind (Fig. 2a, b). In Poland, G. glabra prefers dense grasses in the lower positions 10 cm above the ground. Vacant sites were based mainly on dense grasses reaching usually 50–60 cm, sometimes even 1–1.2 m in height, with the exception of two flowering plants: Galium verum and Thalictrum minus (Fig. 5). The degree of vegetation cover at a height of 40 cm and cover with meadow plants were both factors that were negatively associated with the occurrence of G. glabra. This is contrary to the results of other authors. In Slovakia, G. glabra prefers dense grassy and herbal stands taller than 50 cm (Krištín et al. 2007), while in Moldova it lives in grassland where the height of grasses reaches about 40–60 cm (Stahi and Derjanschi 2011). In this study, some of the grass species dominating squares that were not occupied by G. glabra are most commonly found on flood plains (Fig. 5). The examples are: Agrostis stolonifera, Dactylis glomerata and Deschampsia caespitosa. This is consistent with the results of Krištín et al. (2007, 2011) concerning dunes occupied by G. glabra surrounded by wet depressions. In order to confirm this, however, it would be necessary to supplement the research with aspects of landform features and information on soil types and depths of groundwater.
The interesting preference for a dense sward at a height of 10 cm demonstrated in Poland may be related to the need to hide in the event of danger. This may be quite important for a relatively low mobile insect that has limited possibilities to escape and may need shelter. The second reason may be a potentially lower temperature in dense vegetation near the ground, giving cover in the event of dangerously high temperatures on a sunlit, heated hill, although G. glabra is considered to be thermophilous. A thinning sward at 40 cm above ground may be, however, associated with better acoustic communication. Dense and tall plants suppress acoustics (Bennet-Clark 1998; Gardiner et al. 2005), and acoustic communication can be easier when the vegetation is sparse. It is difficult to say how much the choice of such physiognomy resulted from its functionality for the study species. It may also just be the result of the construction of a plant community from the Festuco-Brometea class. The flowering grasslands from this class supplemented with few grass species are rather sparse and densest down above the ground.
Our study confirms the established fact that Orthoptera are sensitive to discontinuity and changes in the environment. Therefore, they can be treated as bioindicators (Riede 1998; Fartmann et al. 2012; Kenyeres et al. 2020). They are also ideal organisms to study occurrence and abundance in the context of environmental characteristics. They are easy to monitor and respond quickly to habitat changes, even within 1 year (Pasinelli et al. 2013). Fragmentation of habitats disturbing the integrity of the population is a serious threat to Orthoptera species (Zschokke et al. 2000). Loss of xerothermic meadows and lower habitat diversity are causes of the decline of Orthoptera in agricultural landscapes (Marini et al. 2010). A strict preference for xerothermic habitat may turn out to be fatal for G. glabra in the future on the studied patch of habitat.
Habitat fragmentation can have, however, various effects on different orthopterans. In Germany, the abundance of Pseudochorthippus parallelus is negatively affected by the heterogeneity of landscape. This trend is stronger in the case of medium and heavily used areas compared to those less intensively used (Wiesner et al. 2014). In contrast, after experimental fragmentation in Switzerland, P. albopunctata—the most efficient flier among the studied Orthopterans—occurs more frequently in fragments (Zschokke et al. 2000). In addition, the role of the matrix in which the fragments are located should not be omitted (Prugh et al. 2008). The network of links between patches allowing movement of species reduces the risk of their extinction (Steffan-Dewenter and Tscharntke 2002). Sometimes, however, it decreases population density in a patch when insects leave it through terrain with optimal vegetation, as in the case of Saga pedo (Holuša et al. 2013). In the future, the researched patch of habitat should be prevented from separating into xerothermic parts and areas of meadows with grazing or mowing regimes and the best state of preservation of xerothermophilous plants should be ensured.
Conclusions and conservation implications
The disappearance of vegetation with the physiognomy of steppes and strict habitat specialization of G. glabra probably contributed to the decline of most locations of this species in Poland and may contribute to the decline in the abundance of this species in central and western Europe. The cutting or grazing of habitats, divided into sectors, and reintroducing extensive sheep grazing into valuable xerothermic locations are suggested as complementary strategies to mitigate the possible further decline of G. glabra in Poland. In the case of the threat to isolated populations, translocation of insect species is promising, as long as the release localities contain sufficiently large areas of suitable habitat (Hochkirch et al. 2007). Unfortunately, colonization success decreases with increasing isolation (Kruess and Tscharntke 2000). This may be a problem regarding the possible transfer of G. glabra living in isolated patches in many countries of Europe.
Another purpose of this work was to indicate to what extent a strict and relatively low mobile habitat specialist modifies its habitat preferences after a long period of isolation. The results suggest that despite the long-term isolation of the study area, G. glabra still maintains its strict habitat specialization. Accurate characterization of patches of the xerothermic habitats occupied by G. glabra, like the one in this paper, allows the development of a population protection strategy. The study of habitat requirements of the endangered population in this isolated location could serve as a prelude to the restoration of similar locations potentially useful for the species before it becomes locally extinct. This study may be also an introduction to the development of a conservation strategy for the heath bush-cricket on a global scale.
References
Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Bennet-Clark HC (1998) Size and scale effects as constraints in insect sound communication. Phil Trans R Soc Lond B 353:407–419
Berg H-M, Zuna-Kratky T (1997) Heuschrecken und Fangschrecken. Eine Rote Liste der in Niederösterreich gefährdeten Arten. NÖ Landesregierung, Wien
Collinge SK (2000) Effects of grassland fragmentation on insect species loss, colonization, and movement patterns. Ecology 81(8):2211–2226
Fartmann T, Krämer B, Stelzner F, Poniatowski D (2012) Orthoptera as ecological indicators for succession in steppe grassland. Ecol Indic 20:337–344
Fekete G, Molnár Z, Magyari E, Somodi I. Varga Z (2014) A new-framework for understanding Pannonian vegetation patterns: regularities, deviations and uniqueness. Community Ecol 15:12–26
Fischer FP, Schulz U, Schubert H, Knapp P, Schmöger M (1997) Quantitative assessment of grassland quality: acoustic determination of population sizes of orthopteran indicator species. Ecol Appl 7:909–920
Gardiner T (2010) Hedgerow species richness influences the presence of Orthoptera and Dermaptera along green lanes in Essex, UK. Entomol Gaz 61:53–64
Gardiner T (2018) Grazing and Orthoptera: a review. J Orthop Res 27(1):3–11
Gardiner T, Hill J, Chesmore D (2005) Review of the methods frequently used to estimate the abundance of Orthoptera in grassland ecosystems. J Insect Conserv 9:151–173
Hartley JC (1990) Egg biology of the Tettigoniidae. In: Bailey WJ, Rentz DCF (eds) The Tettigoniidae, biology, systematics and evolution. Crawford House Press, Bathurst, pp 41–70
Helversen von D (1972) Gesang des Männchens und Lautschema des Weibchens bei der Feldheuschrecke Chorthippus biguttulus (Orthoptera, Acrididae). J Comp Physiol A 81:381–422
Hochkirch A (2014) Gampsocleis glabra (errata version published in 2017). The IUCN Red List of Threatened Species 2014: e.T44711951A115472224. https://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T44711951A53872077.en. Accessed 10 Feb 2018
Hochkirch A, Witzenberger KA, Teerling A, Niemeyer F (2007) Translocation of an endangered insect species, the field cricket (Gryllus campestris Linnaeus, 1758) in northern Germany. Biodivers Conserv 16:3597–3607
Holuša J (2012) Grasshoppers and bush crickets regionally extinct in the Czech Republic: consequence of the disappearance of habitats scattered on the edge of their ranges. J Insect Conserv 16:949–960
Holuša J, Kočárek P, Vlk R (2013) Monitoring and conservation of Saga pedo (Orthoptera: Tettigoniidae) in an isolated northwestern population. J Insect Conserv 17:663–669
Imdadullah M, Aslam M, Altaf S (2016) mctest: an R package for detection of collinearity among regressors. R J 8/2:495–505
Ingrisch S, Köhler G (1998) Die Heuschrecken Mitteleuropas. Die Neue Brehm Bücherei 629. Westarp Wissenschaften, Magdeburg
Iorgu I, Iorgu E (2008) Bush-crickets and Grasshoppers from Moldavia (Romania). Edit Pim Iaşi
Kajtoch Ł (2011) Conservation genetics of xerothermic beetles in Europe: the case of Centricnemus leucogrammus. J Insect Conserv 15:787–797
Kajtoch Ł, Mazur M, Kubisz D, Mazur MA, Babik W (2014) Low effective population sizes and limited connectivity in xerothermic beetles: implications for the conservation of an endangered habitat. Anim Conserv 17:454–466
Kajtoch Ł, Cieślak E, Varga Z, Paul W, Mazur MA, Sramkó G, Kubisz D (2016) Phylogeographic patterns of steppe species in Eastern Central Europe: a review and the implications for conservation. Biodivers Conserv 25:2309–2339
Kassambara A, Mundt F (2019) factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.6. http://www.sthda.com/english/rpkgs/factoextra. Accessed 7 Feb 2020
Kenyeres Z, Szabó S, Takács G, Szinetár C (2020) Orthoptera assemblages as indicators for the restoration of sand grassland networks. North-West J Zool 16(1):7–14
Kostuch R, Misztal A (2006) Occurrence of xerothermic vegetation in the Małopolska Upland. Infrastruct Ecol Rural Areas 4(3):1363–1375
Krauss J, Bommarco R, Guardiola M, Heikkinen RK, Helm A, Kuussaari M, Lindborg R, Öckinger E, Pärtel M, Pino J, Pöyry J, Raatikainen KM, Sang A, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2010) Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett 13:597–605
Krausz K, Pápai J, Gallé L (1995) Composition of Orthoptera assemblages in grassland habitats at Lower-Tisza flood plain. Tiscia 29:47–52
Krištín A, Balla M, Fabriciusová V, Hrúz V, Kaňuch P (2011) Orthoptera and Mantodea in fragments of seminatural habitats in lowlands of SE Slovakia and SW Transcarpathian Ukraine. Articulata 26(2):109–121
Krištín A, Gavlas V, Balla M, Kaňuch P (2004) Orthoptera and Mantodea of the East-Slovakian lowland (Východoslovenská nížina). Folia Entomol Hung 65:43–54
Krištín A, Kaňuch P, Balla M, Gavlas V (2007) On distribution and ecology of Gampsocleis glabra and Tettigonia caudata (Orthoptera) in Slovakia. Articulata 22(1):1–10
Kruess A, Tscharntke T (2000) Species richness and parasitism in a fragmented landscape: experiments and field studies with insects on Vicia sepium. Oecologia 122:129–137
Latimer W (1980) Song and spacing in Gampsocleis glabra (Orthoptera, Tettigoniidae). J Nat Hist 14(2):201–213
Le S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Soft 25(1):1–18
Lengyel S, Déri E, Magura T (2016) Species richness responses to structural or compositional habitat diversity between and within grassland patches: a multi-taxon approach. PLoS ONE 11(2):e0149662
Liana A (1976) Orthoptera of the xerothermic habitats on the Małopolska Upland. Fragm Faun 20:469–558
Liana A (2007) Gampsocleis glabra (Herbst, 1786), Stepówka, Heath Bush-cricket. Polish red data book of animals—invertebrates. Institute of Nature Conservation Polish Academy of Science, Kraków
Łuszczyńska B (1998) Xerothermic vascular flora of selected subregions of Nida Basin (Pińczów Mountains, Szaniec Plateau, eastern part of Solec Basin). Fragm Flor Geobot Pol 5:55–87
Maas S, Detzel P, Staud A (2002) Gefährdungsanalyse der Heuschrecken Deutschlands. Verbreitungsatlas, Gefährdungseinstufung und Schutzkonzepte. Bundesamt für Naturschutz, Bonn- 513 Bad Godesberg, Bonn
Marini L, Bommarco R, Fontana P, Battisti A (2010) Disentangling effects of habitat diversity and area on orthopteran species with contrasting mobility. Biol Conserv 143(9):2164–2171
Markova AK, Simakova AN, Puzachenko AY (2009) Ecosystems of Eastern Europe at the time of maximum cooling in the Valdai glaciation (24–18 kyr BP) inferred from data on plant communities and mammal assemblages. Quat Int 201:53–59
Matuszkiewicz W (2005) Guide for identification plant communities in Poland. PWN, Warsaw
Medwecka-Kornaś A, Kornaś J (1972) Associations of steppes and dry grasslands. Plant Commun Pol 1:352–366
Mirek Z, Piękoś-Mirkowa H, Zając A, Zając M (2002) Flowering plants and pteridophytes of Poland: a checklist. Biodiversity of Poland. Szafer Institute of Botany, Polish Academy of Sciences, Kraków
Misztal A, Zarzycki J, Bedla D (2015) Habitat conditions of Sisymbrio-Stipetum capillatae and Koelerio-Festucetum sulcatae steppe plant associations in the Ostoja Nidziańska specially protected area. Infrastruct Ecol Rural Areas 4(3):1363–1375
Pasinelli G, Meichtry-Stier K, Birrer S, Baur B, Duss M (2013) Habitat quality and geometry affect patch occupancy of two Orthopteran species. PLoS ONE 8(5):e65850
Pilskog HE, Birkemoe T, Framstad E, Sverdrup-Thygeson A (2016) Effect of habitat size, quality, and isolation on functional groups of beetles in hollow oaks. J Insect Sci 16(1):1–8
Pokorný P, Chytrý M, Juřičková L, Sádlo J, Novák J, Ložek V (2015) Mid-Holocene bottleneck for central European dry grasslands: did steppe survive the forest optimum in northern Bohemia, Czech Republic? Holocene 25:716–726
Poniatowski D, Fartmann T (2010) What determines the distribution of a flightless bush-cricket (Metrioptera brachyptera) in a fragmented landscape? J Insect Conserv 14:637–645
Poniatowski D, Löffler F, Stuhldreher G, Borchard F, Krämer B, Fartmann T (2016) Functional connectivity as an indicator for patch occupancy in grassland specialists. Ecol Indic 67:735–742
Poniatowski D, Stuhldreher G, Löffler F, Fartmann T (2018) Patch occupancy of grassland specialists: Habitat quality matters more than habitat connectivity. Biol Conserv 225:237–244
Prugh LR, Hodges KE, Sinclair ARE, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. PNAS 105(52):20770–20775
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/index.html. Accessed 3 Jan 2020
Riede K (1998) Acoustic monitoring of Orthoptera and its potential for conservation. J Insect Conserv 2:217–223
Schafer J, Opgen-Rhein R, Zuber V, Ahdesmaki M, Duarte Silva AP, Strimmer K (2017) Corpcor: efficient estimation of covariance and (partial) correlation. R package version 1.6.9. https://CRAN.R-project.org/package=corpcor. Accessed 21 Oct 2019
Schirmel J, Blindow I, Fartman T (2010) The importance of habitat mosaics for Orthoptera (Caelifera and Ensifera) in dry heathlands. Eur J Entomol 107:129–132
Schloerke B, Crowley J, Cook D, Briatte F, Marbach M, Thoen E, Elberg A, Larmarange J (2018) GGally: extension to ‘ggplot2’. R package version 1.4.0. https://CRAN.R-project.org/package=GGally. Accessed 21 Oct 2019
Schouten MA, Verwij PA, Barendregt A, Kleukers RJM, de Ruiter PC (2007) Nested assemblages of Orthoptera species in the Netherlands: the importance of habitat features and life-history traits. J Biogeogr 34(11):1938–1946
Stahi N, Derjanschi V (2011) Rare species of Orthoptera (Insecta) from the Republic of Moldova. Oltenia, Studii si Comunicari Stiintele Naturii 27(2):47–50
Steffan-Dewenter I, Tscharntke T (2002) Insect communities and biotic interactions on fragmented calcareous grasslands—a mini review. Biol Conserv 104:275–284
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc R Soc Lond B 268:1791–1796
Towpasz K (2011) History of the research on xerothermic vegetation in the Nida Basin and problems related to its conservation. Ann Univ Mariae Curie-Skłodowska 66(2):33–43
Towpasz K, Stachurska-Swakoń A (2012) Seslerio uliginosae-Scorzoneretum purpureae (Festuco-Brometea class) in the Nida Basin (Małopolska Upland) after 90 years. Acta Soc Bot Pol 81(3):167–173
Török P, Ambarlı D, Kamp J, Wesche K, Dengler J (2016) Step(pe) up! raising the profile of the palaearctic natural grasslands. Biodivers Conserv 25(12):2187–2195
Vahed K (1994) The Evolution and Function of the Spermatophylax in Bushcrickets (Orthoptera: Tettigoniidae). PhD Thesis, University of Nottingham, UK
Valko O, Żmihorski M, Biurrun I, Loos J, Labadessa R, Venn S (2016) Ecology and conservation of steppes and semi-natural grassland. Hacquetia 15/2:5–14
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wiesner KR, Habel JC, Gossner MM, Loxdale HD, Köhler G, Schneider ARR, Tiedemann R, Weisser W (2014) Effects of habitat structure and land-use intensity on the genetic structure of the grasshopper species Chorthippus parallelus. R Soc Open Sci 1:140133
Willis KJ, van Andel TA (2004) Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat Sci Rev 23:2369–2387
Wilson JB, Peet RK, Dengler J, Pärted M (2012) Plant species richness: the world records. J Veg Sci 23:796–802
Zschokke S, Dolt C, Rusterholz H-P, Oggier P, Braschler B, Thommen GH, Lüdin E, Erhardt A, Baur B (2000) Short-term responses of plants and invertebrates to experimental small-scale grassland fragmentation. Oecologia 125:559–572
Acknowledgements
The authors kindly thank to J.L. León-Cortés, T. Fartmann, two anonymous reviewers and Ł. Kajtoch for valuable comments on the manuscript. The Wildlife Acoustics Product Grant Q1/2018 supported the equipment used in research; E. Grzędzicka was the grant recipient in competition. Title of the project: “Acoustic activity and conservation of the endangered heath bush-cricket Gampsocleis glabra (Orthoptera, Tettigonioidea) on xerothermic habitats in south-eastern Poland”. Many thanks to the Foundation for Silesia Park for organizational support in the project.
Funding
The digital recorder Song Meter SM4 was provided by Wildlife Acoustics Product Grant Q1/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Insects were not caught, touched, or disturbed. Research complies with current laws in Poland and all the necessary permits were obtained for the field study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzędzicka, E., Vahed, K. Habitat requirements of the endangered heath bush-cricket Gampsocleis glabra (Orthoptera, Tettigoniidae) in an isolated population. J Insect Conserv 24, 935–945 (2020). https://doi.org/10.1007/s10841-020-00265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00265-9