Abstract
Purpose
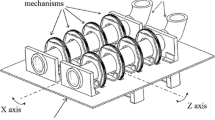
Gastrointestinal hydrodynamics are poorly replicated in vitro and can significantly alter the release kinetics of drug products due to compressive forces in the stomach and peristaltic movement in the intestines. In this work, we describe the development and application of a predictive in vitro dissolution device that simulates gastrointestinal forces for the testing of oral drug products. The peristaltic dissolution device developed herein is designed as an addition to the common USP Apparatus 2 that applies repetitive compressive forces via a piston during dissolution testing of a product to replicate in vivo conditions.
Methods
A dissolution testing device was designed, fabricated, and evaluated against human in vivo pharmacokinetic data to better mimic the physical forces present in the gastrointestinal tract. An optimized compression protocol to predict in vivo dissolution was developed using clinical data from two modified release carvedilol drug products. The apparatus was further evaluated using data from an additional modified release drug product. Finally, additional dissolution studies were performed to evaluate the utility of the apparatus for in vitro analysis of medicated gums, gastric retentive formulations, and long-acting injectable drug depots.
Results
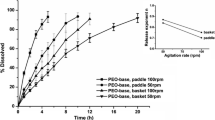
The device was successfully implemented and the protocol to use the device was optimized using two initial drug products and further evaluated using an additional three drug products. The optimized protocol included a 1-h lag time (applicable in the fed state), followed by a cycle of 3 s of compression with 6 s intervals between compressions. Additional applications of the peristaltic dissolution device were also demonstrated through small exploratory studies, with continued potential for further optimization of the testing protocols following further research.
Conclusion
This simple compressive device referred to as the “peristaltic dissolution device” was successfully proven to better predict in vivo performance of modified release drug products, as gastrointestinal mechanical forces have been observed to significantly impact and occasionally cause complete dose dumping of controlled release formulations. In addition, it has proven to be easily adapted for evaluation of other drug products such as medicated gums, gastric retentive formulations, and ex vivo long-acting injectable drug depots.















Similar content being viewed by others
Data Availability
Clinical data used in this manuscript has been previously published and citations to the published data are included herein.
Abbreviations
- API:
-
Active pharmaceutical ingredient
- BSA:
-
Bovine serum albumin
- CTAB:
-
Cetyltrimethylammonium bromide
- GI:
-
Gastrointestinal
- GSK:
-
GlaxoSmithKline
- IVIVC:
-
In vitro–in vivo correlation
- LOD:
-
Limit of detection
- PBS:
-
Phosphate buffered saline
- PEG:
-
Polyethylene glycol
- PK:
-
Pharmacokinetics
- QbD:
-
Quality by design
- TNO TIM-1:
-
TNO [organization name] intestinal model 1
- UHPLC/MS:
-
Ultra-high performance liquid chromatography–mass spectrometry
- USP:
-
United States Pharmacopeia
References
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66.
Reppas C, Vertzoni M. Biorelevant in-vitro performance testing of orally administered dosage forms. J Pharm Pharmacol. 2012;64:919–30.
Garbacz G, Klein S. Dissolution testing of oral modified-release dosage forms. J Pharm Pharmacol. 2012;64:944–68.
Cassilly D, Kantor S, Knight LC, Maurer AH, Fisher RS, Semler J, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–9.
Simmons DL, Legore AA, Picotte P, Lee KS, Joshi NN. A dissolution rate apparatus for the prediction of initial drug absorption patterns in beagles: tolbutamide tablets. J Pharmacokinet Biopharm. 1975;3:39–49.
Simmons DL. Peristaltic dissolution assembly as a bioequivalence surrogate: a review. 2016.https://doi.org/10.13140/RG.2.1.2436.6961
Koziolek M, Görke K, Neumann M, Garbacz G, Weitschies W. Development of a bio-relevant dissolution test device simulating mechanical aspects present in the fed stomach. Eur J Pharm Sci. 2014;57:250–6.
Garbacz G, Wedemeyer R-S, Nagel S, Giessmann T, Mönnikes H, Wilson CG, et al. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur J Pharm Biopharm. 2008;70:421–8.
Gao Z, Ngo C, Ye W, Rodriguez JD, Keire D, Sun D, et al. Effects of dissolution medium pH and simulated gastrointestinal contraction on drug release from nifedipine extended-release tablets*. J Pharm Sci. 2019;108:1189–94.
Minekus M. The TNO Gastro-Intestinal Model (TIM). In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al., editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models. Cham: Springer International Publishing. 2015;37–46.https://doi.org/10.1007/978-3-319-16104-4_5
Verwei M, Freidig AP, Havenaar R, Groten JP. Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J Nutr. 2006;136:3074–8.
Mateo Anson N, Havenaar R, Bast A, Haenen GRMM. Antioxidant and anti-inflammatory capacity of bioaccessible compounds from wheat fractions after gastrointestinal digestion. J Cereal Sci. 2010;51:110–4.
Larsson M, Minekus M, Havenaar R. Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model. J Sci Food Agric. 1997;74:99–106.
Krul C, Luiten-Schuite A, Baan R, Verhagen H, Mohn G, Feron V, et al. Application of a dynamic in vitro gastrointestinal tract model to study the availability of food mutagens, using heterocyclic aromatic amines as model compounds. Food Chem Toxicol. 2000;38:783–92.
Naylor TA, Connolly PC, Martini LG, Elder DP, Minekus M, Havenaar R, et al. Use of a gastro-intestinal model and Gastroplus™ for the prediction of in vivo performance. J Appl Ther Res. 2006;6:15.
Kong F, Singh RP. Disintegration of solid foods in human stomach. J Food Sci. 2008;73:R67–80.
Burke M, Coffin M, Lamey K, Martini L, Oh C, Peterson H, et al. Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods. 2006. Available from: https://patents.google.com/patent/US20060182804A1/en?oq=us20060182804a1.
Burke MD, Maheshwari CR, Zimmerman BO. Pharmaceutical analysis apparatus and method [Internet]. 2006. Available from: https://patents.google.com/patent/WO2006052742A2/en?oq=WO2006052742.
Burke M, Beato S, Barish P, Doucet D, Casazza A, Coffin M. Advanced in-vitro dissolution apparatus: novel peristaltic dissolution and TNO TIM-1. 2004.
Henderson LS, Tenero DM, Campanile AM, Baidoo CA, Danoff TM. Ethanol does not alter the pharmacokinetic profile of the controlled-release formulation of carvedilol. J Clin Pharmacol. 2007;47:1358–65.
Johnson M, Jewell RC, Peppercorn A, Gould E, Xu J, Lou Y, et al. The safety, tolerability, and pharmacokinetic profile of GSK2838232, a novel 2nd generation HIV maturation inhibitor, as assessed in healthy subjects. Pharmacol Res Perspect. 2018;6:e00408.
George JK, Singh SK, Verma P. In vivo in silico pharmacokinetic simulation studies of carvedilol-loaded nanocapsules using GastroPlus™. Ther Deliv. 2016;7:305–18.
Langenbucher F. Handling of computational in vitro/in vivo correlation problems by Microsoft Excel: III. Convolution and deconvolution. Eur J Pharm Biopharm. 2003;56:429–37.
Tambwekar KR, Kakariya RB, Garg S. A validated high performance liquid chromatographic method for analysis of nicotine in pure form and from formulations. J Pharm Biomed Anal. 2003;32:441–50.
Burke M. Transforming the patient experience through long acting injectable/implantable formulations: new opportunities and technologies [Internet]. Washington, DC; 2018. Available from: https://www.researchgate.net/publication/331044264_Keynote_Speaker_Transforming_the_Patient_Experience_through_Long_Acting_InjectableImplantable_Formulations_New_Opportunities_and_Technologies.
Abrahamsson B, Roos K, Sjögren J. Investigation of prandial effects on hydrophilic matrix tablets. Drug Dev Ind Pharm. 1999;25:765–71.
Todaro V, Persoons T, Grove G, Healy AM, D’Arcy DM. Characterization and simulation of hydrodynamics in the paddle, basket and flow-through dissolution testing apparatuses - a review. Dissolution Technol. 2017;24:24–36.
Maderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154:2–19.
Gajendran J, Kraemer J, Knudsen SR. Product performance test for medicated chewing gums. Dissolution Technol. 2010;17:15–8.
Kvist C, Andersson SB, Fors S, Wennergren B, Berglund J. Apparatus for studying in vitro drug release from medicated chewing gums. Int J Pharm. 1999;189:57–65.
Morjaria Y, Irwin WJ, Barnett PX, Chan RS, Conway BR. In Vitro Release of Nicotine From Chewing Gum Formulations. Dissolution Technol. 2004;11:12–5
Yang X, Wang G, Zhang X. Release kinetics of catechins from chewing gum. J Pharm Sci. 2004;93:293–9.
Faraj JA, Dorati R, Schoubben A, Worthen D, Selmin F, Capan Y, et al. Development of a peptide-containing chewing gum as a sustained release antiplaque antimicrobial delivery system. AAPS PharmSciTech. 2007;8:E177–85.
Nemeth-Coslett R, Benowitz NL, Robinson N, Henningfield JE. Nicotine gum: chew rate, subjective effects and plasma nicotine. Pharmacol Biochem Behav. 1988;29:747–51.
Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, et al. Oral, ultra–long-lasting drug delivery: application toward malaria elimination goals. Sci Transl Med. 2016;8:365ra157.
Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J Control Release. 2003;90:143–62.
Anderson FD, Archer DF, Harman SM, Leonard RJ, Wilborn WH. Tissue response to bioerodible, subcutaneous drug implants: a possible determinant of drug absorption kinetics. Pharm Res. 1993;10:369–80.
Darville N, van Heerden M, Vynckier A, De Meulder M, Sterkens P, Annaert P, et al. Intramuscular administration of paliperidone palmitate extended-release injectable microsuspension induces a subclinical inflammatory reaction modulating the pharmacokinetics in rats. J Pharm Sci. 2014;103:2072–87.
Darville N, van Heerden M, Mariën D, De Meulder M, Rossenu S, Vermeulen A, et al. The effect of macrophage and angiogenesis inhibition on the drug release and absorption from an intramuscular sustained-release paliperidone palmitate suspension. J Control Release. 2016;230:95–108.
Acknowledgments
The authors would like to acknowledge the Jeff Seely and Distek team for continuing development work on the Peristaltic Dissolution Device, as well as David Curran, Stefania Beato, Nena Mistry, Paul Connolly, Richard Lloyd, Lihua Zhang, Philip Barish, Brian Zimmerman, Mark Coffin, Alan Parr, and David DeMagistris for their technical expertise and work in support of this project.
Funding
Research was funded and performed via GlaxoSmithKline. No external funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burke, M.D., Koetting, M.C. Development of a Clinically Relevant Dissolution Approach to Simulate Physiological Forces with a USP 2 Apparatus: “Peristaltic Dissolution”. J Pharm Innov 16, 699–714 (2021). https://doi.org/10.1007/s12247-020-09485-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09485-7