Abstract
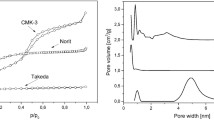
Indole-3-acetic acid (IA, urine-like toxin) coexisting with atorvastatin (AT) was adsorbed by spherical carbonaceous adsorbents (SCs). The relationship between the competitive adsorption kinetics and the mesoporous structures was investigated. The pore structures of the four SCs were analyzed using the Hg and gas adsorption methods. SC adsorption tests were performed using the dissolution test with the paddle method with an SC sample in distilled water containing IA and/or AT at 37.0 °C. The amounts of IA and AT adsorbed on SCs in single- and binary-drug administration tests were measured based on the ultraviolet-visible spectra using classical least squares analysis. Rate constants for the drug adsorption of IA and AT by SCs (kIA and kAT, respectively) were suppressed by the effect of coexisting drugs. The suppression of IA adsorption by the coexisting drug was dependent on the pore structure of the SCs. kIA and kAT against the SCs were competitively inhibited by coexisting drugs.

Graphical abstract













Similar content being viewed by others
References
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem Eng 6(4):4676–4697
Kyzas GZ, Fu J, Lazaridis NK, Bikiaris DN, Matis KA (2015) New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J Mol Liq 209:87–93
Rúa-Gómez PC, Püttmann W (2012) Occurrence and removal of lidocaine, tramadol, venlafaxine, and their metabolites in German wastewater treatment plants. Environ Sci Pollut Res Int 19:689–699. https://doi.org/10.1007/s11356-011-0614-1
Kim SH, Shon HK, Ngo HH (2010) Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J Ind Eng Chem 16:344–349. https://doi.org/10.1016/j.jiec.2009.09.061
Masson S, Gineys M, Delpeux-Ouldriane S, Reinert L, Guittonneau S, Béguin F, Duclaux L (2016) Single, binary, and mixture adsorption of nine organic contaminants onto a microporous and a microporous/mesoporous activated carbon cloth. Microporous Mesoporous Mater 234:24–34
Mansouri H, Carmona RJ, Gomis-Berenguer A, Souissi-Najar S, Ouederni A, Ania CO (2015) Competitive adsorption of ibuprofen and amoxicillin mixtures from aqueous solution on activated carbons. J Colloid Interface Sci 449:252–260
Honda Y, Nakano N, Nakano Y (1985) Activated carbon beads as an emergency detoxifier. Farumashia 21(5):414–417
Niwa T (2010) Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci 72(1–2):1–11
Honda Y, Nakano M (1994) Studies of the pharmacological interaction of Kremezin capsule 200 with drugs. Clin Rep 28:2873–2881
Honda Y, Nakano M (1997) Studies on the adsorption characteristics of spherical charcoal (Kremezin). Jpn J Hosp Pharm 23:219–224
Koide K, Tohyama J, Inoue N (1985) Oral adsorbents. Hemodialysis, Nihon Rinsho, 43, Suppl. 422–440
Yanagawa C, Sakashita Y, Kanzaki Y (2005) Comparison and examination on the physicochemical property between Kremezin and Merckmezin. Pharm Regul Sci 36:497–504
Leaflet of Kuremezin, Pharmaceutical and Medical Device Agency, http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/250052_3929003C1067_1_04 (3/2/2017)
Leaflet of Liptol, Pharmaceutical and Medical Device Agency, http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/800126_2189015F1023_1_32 (3/2/2017)
Merck Seiyaku KK (2004) Package insert of Merckmezin
Mylan Seiyaku KK (2009) Package insert of SCA Mylan. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/3929003 (3/20/2009)
Abe H, Morikawa R, Otsuka M (2013) Adsorption kinetics and mechanistic studies for pharmaceutical spherical carbon adsorbents: comparison of a brand product and two genetics. Colloids Surf B 103:538–554
Abe H, Otsuka M (2012) Comparison of physico-chemical characteristics among three pharmaceutical spherical carbon adsorbents. Colloids Surf B 100:90–94
Hattori Y, Nitta Y, Otsuka M (2017) Effect of coexisting atorvastatin calcium on in vitro uremic-like-toxin adsorption in gastrointestinal tract model solution by spherical carbon adsorbent for chronic renal failure therapy. J Drug Delivery Sci Technol 39:484–489. https://doi.org/10.1016/j.jddst.2017.03.006
Indole3acetic acid (IAA), www.chemicalland21.com, http://www.chemicalland21.com/lifescience/agro/INDOLE-3-ACETIC%20ACID.htm (11/7/2016)
Sing KS (2004) Characterization of porous materials: past, present and future. Colloids Surf A Physicochem Eng Asp 241(1–3):3–7
Schröder M, Kleinebudde P (1995) Structure of disintegrating pellets with regard to fractal geometry. Pharm Res 12:1694–1700
Usteri M, Bonny JD, Leuenberger H (1990) Fractal dimension of porous solid dosage forms. Pharm Acta Helv 65:55
Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N (2007) A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal 44(3):683–700
Thommes M, Cychosz KA (2014) Physical adsorption characterization of nanoporous materials: progress and challenges. Adsorption 20(2–3):233–250
Sher P, Ingavle G, Ponrathnam S, Pawar AP (2007) Low density porous carrier: drug adsorption and release study by response surface methodology using different solvents. Int J Pharm 331(1):72–83
Masel RI (1996) Principles of adsorption and reaction on solid surfaces (Vol. 3). Wiley. p. 240. ISBN 978-0-471-30392-3
Skinner LM, Sambles JR (1972) The Kelvin equation—a review. J Aerosol Sci 3(3):199–210
Shibata T, Nomura Y, Takada A, Ueno M, Katashima M, Yazawa R, Furihata K (2019) Evaluation of food and spherical carbon adsorbent effects on the pharmacokinetics of roxadustat in healthy nonelderly adult male Japanese subjects. Clin Pharmacol Drug Dev 8(3):304–313
Funding
This research received partial financial support from the Creating Happiness Incubation, Musashino University (No. 23).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nitta, Y., Hattori, Y. & Otsuka, M. Effect of mesoporous characteristics on competitive adsorption kinetics of uremic-like toxin and atorvastatin on spherical carbon adsorbents for an in vitro chronic renal failure therapeutic model. Colloid Polym Sci 298, 1487–1500 (2020). https://doi.org/10.1007/s00396-020-04729-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-020-04729-x