Abstract
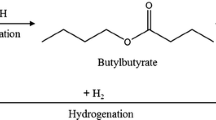
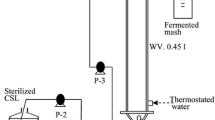
Butyric acid, a short chain carboxylic acid with diverse usages, is produced by Clostridia fermentation. In the industrial scale production of butyric acid, its separation and recovery from fermentation broth requires energy-intensive processes. To reduce the product recovery costs, it is necessary to convert butyric acid into a chemical with a much higher partition coefficient in the hydrophobic extractants than butyric acid. Butyl butyrate, with an excellent partition coefficient to tetradecane, can be produced by the enzymatic conversion of butyric acid via esterification. Moreover, butyl butyrate can be used as a valuable fuel source and additive in the food, cosmetic, and pharmaceutical industries. Novozyme 435, Candida antarctica lipase B immobilized on acrylic resin, and tetradecane were used as the enzyme and extractant, respectively. A high-pressure CO2-facilitated reactor was used to temporarily drop the pH of the fermentation broth so that the enzymatic reaction could be activated. The in situ removal of butyric acid and simultaneous production of butyl butyrate were processed continuously during fermentation. To optimize the enzymatic reaction, it was necessary to maintain a temperature of 40°C at 50 bar and an optimal molar ratio of substrate. In the extractive fermentation, 11.6 g/L butyl butyrate was produced with a productivity of 0.77 g/L/h from butyrate produced through fermentation. This process is expected to be able to extract carboxylic acids with more diverse carbon lengths in ester form.
Similar content being viewed by others
References
Sathesh-Prabu, C., K. S. Shin, G. H. Kwak, S. K. Jung, and S. K. Lee (2019) Microbial production of fatty acid via metabolic engineering and synthetic biology. Biotechnol. Bioprocess Eng. 24: 23–40.
Rhie, M. N., H. T. Kim, S. Y. Jo, L. L. Chu, K. A. Baritugo, M. G. Baylon, J. Lee, J. G. Na, L. H. Kim, T. W. Kim, C. Park, S. H. Hong, J. C. Joo, and S. J. Park (2019) Recent advances in the metabolic engineering of Klebsiella pneumoniae: A potential platform microorganism for biorefineries. Biotechnol. Bioprocess Eng. 24: 48–64.
Park, S., H. Kim, S. Cho, G. You, H. B. Oh, J. H. Han, and J. Lee (2019) Enhanced incorporation of gaseous CO to succinate by a recombinant Escherichia coli W3110. Biotechnol. Bioprocess Eng. 24: 103–108.
Sjoblom, M., L. Matsakas, P. Christakopoulos, and U. Rova (2016) Catalytic upgrading of butyric acid towards fine chemicals and biofuels. FEMS Microbiol. Lett. 363: fnw064.
Severini, F., T. Flannelly, D. O. Nolan, J. J. Leahy, and W. Kwapinski (2016) Development of heterogeneous acid catalysts produced from the carbonization of Miscanthus x giganteus for the esterification of butyric acid to butyl butyrate with n-butanol. J. Chem. Technol. Biotechnol. 91: 2076–2084.
Jha, A. K., J. Li, Y. Yuan, N. Baral, and B. Ai (2014) A review on bio-butyric acid production and its optimization. Int. J. Agric. Biol. 16: 1019–1024.
Sjöblom, M., L. Matsakas, P. Christakopoulos, and U. Rova (2015) Production of butyric acid by Clostridium tyrobutyricum (ATCC25755) using sweet sorghum stalks and beet molasses. Ind. Crops Prod. 74: 535–544.
Chun, J., O. Choi, and B. I. Sang (2018) Enhanced extraction of butyric acid under high-pressure CO conditions to integrate chemical catalysis for value-added chemicals and biofuels. Biotechnol. Biofuels. 11: 119.
Dwidar, M., J. Y. Park, R. J. Mitchell, and B. I. Sang (2012) The future of butyric acid in industry. ScientificWorldJournal. 2012: 471417.
Jiang, L., J. Wang, S. Liang, J. Cai, Z. Xu, P. Cen, S. Yang, and S. Li (2011) Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol. Bioeng. 108: 31–40.
Outram, V., C. A. Lalander, J. G. M. Lee, E. T. Davies, and A. P. Harvey (2017) Applied in situ product recovery in ABE fermentation. Biotechnol. Prog. 33: 563–579.
Peterson, E. C. and A. J. Daugulis (2014) Demonstration of in situ product recovery of butyric acid via CO2-facilitated pH swings and medium development in two-phase partitioning bioreactors. Biotechnol. Bioeng. 111: 537–544.
Hwang, H. T., F. Qi, C. Yuan, X. Zhao, D. Ramkrishna, D. Liu, and A. Varma (2014) Lipase-catalyzed process for biodiesel production: protein engineering and lipase production. Biotechnol. Bioeng. 111: 639–653.
Chuck, C. J. and J. Donnelly (2014) The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy. 118: 83–91.
Knothe, G. (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels. 22: 1358–1364.
Jenkins, R. W., M. Munro, S. Nash, and C. J. Chuck (2013) Potential renewable oxygenated biofuels for the aviation and road transport sectors. Fuel. 103: 593–599.
van den Berg, C., A. S. Heeres, L. A. van der Wielen, and A. J. J. Straathof (2013) Simultaneous Clostridial fermentation, lipase-catalyzed esterification, and ester extraction to enrich diesel with butyl butyrate. Biotechnol. Bioeng. 110: 137–142.
Sun, S., L. Tian, B. Hu, and C. Jiang (2018) Enzymatic synthesis of lipophilic caffeoyl lipids using soybean oil as the novel acceptor. Biotechnol. Bioprocess Eng. 23: 557–563.
Zhao, J. F., W. Tao, J. P. Lin, L. R. Yang, and M. B. Wu (2019) Preparation of high-purity 1,3-diacylglycerol using performance-enhanced lipase immobilized on nanosized magnetite particles. Biotechnol. Bioprocess Eng. 24: 326–336.
Zhang, Z. T., S. Taylor, and Y. Wang (2017) In situ esterification and extractive fermentation for butyl butyrate production with Clostridium tyrobutyricum. Biotechnol. Bioeng. 114: 1428–1437.
Seo, S. O., Y. Wang, T. Lu, Y. S. Jin, and H. P. Blaschek (2017) Characterization of a Clostridium beijerinckii spo0A mutant and its application for butyl butyrate production. Biotechnol. Bioeng. 114: 106–112.
Cha, H. J., J. B. Park, and S. Park (2019) Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica lipase b and subtilisin. Biotechnol. Bioprocess Eng. 24: 41–47.
Qureshi, N. and H. P. Blaschek (1999) Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenergy. 17: 175–184.
dos Santos, P., G. L. Zabot, M. A. A. Meireles, M. A. Mazutti, and J. Martínez (2016) Synthesis of eugenyl acetate by enzymatic reactions in supercritical carbon dioxide. Biochem. Eng. J. 114: 1–9.
Caussette, M., A. Gaunand, H. Planche, and B. Lindet (1998) Enzyme inactivation by inert gas bubbling. Prog. Biotechnol. 15: 393–398.
Hernandez-Martin, E. and C. Otero (2008) Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 99: 277–286.
Yadav, G. D. and K. M. Devi (2004) Kinetics of hydrolysis of tetrahydrofurfuryl butyrate in a three phase system containing immobilized lipase from Candida antarctica. Biochem. Eng. J. 17: 57–63.
Delhomme, C., S. L. M. Goh, F. E. Kühn, and D. Weuster-Botz (2012) Esterification of bio-based succinic acid in biphasic systems: Comparison of chemical and biological catalysts. J. Mol. Catal. B Enzym. 80: 39–47.
Zenevicz, M. C. P., A. Jacques, M. J. A. Silva, A. Furigo, V. Oliveira, and D. de Oliveira (2018) Study of a reactor model for enzymatic reactions in continuous mode coupled to an ultrasound bath for esters production. Bioprocess Biosyst. Eng. 41: 1589–1597.
Torres, C. and C. Otero (1999) Part I. Enzymatic synthesis of lactate and glycolate esters of fatty alcohols. Enzyme Microb. Technol. 25: 745–752.
Park, D. W., S. Haam, I. S. Ahn, T. G. Lee, H. S. Kim, and W. S. Kim (2004) Enzymatic esterification of beta-methylglucoside with acrylic/methacrylic acid in organic solvents. J. Biotechnol. 107: 151–160.
Xin, F., A. Basu, K. L. Yang, and J. He (2016) Strategies for production of butanol and butyl-butyrate through lipase-catalyzed esterification. Bioresour. Technol. 202: 214–219.
Acknowledgements
This work was supported in part by a grant funded by Hanyang University in the Republic of Korea (HY-201100000000233-N) and by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20173010092510). The authors declare no conflict of interest. Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chun, J., Sang, BI. Enzymatic Esterification under High-pressure CO2 Conditions for in situ Recovery of Butyric Acid from Anaerobic Fermenters. Biotechnol Bioproc E 25, 616–622 (2020). https://doi.org/10.1007/s12257-020-0158-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-020-0158-7