Abstract
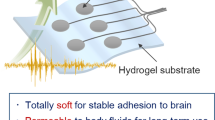
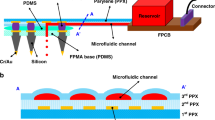
A totally transparent subdural electrode was developed by embedding a conductive poly (vinyl alcohol) (PVA)-filled microchannel made of poly(dimethylsiloxane) (PDMS) into an another PVA hydrogel substrate. Tight bonding between the PVA substrate and the PDMS microchannel (salt bridge) was achieved by mechanical interlocking utilizing the microprotrusions formed on the microchannel. This simple method of bonding without the use of any additives such as silane molecules or nanofibers is very suitable for constructing biomedical devices. The salt bridge electrode (total thickness, ca. 1.5 mm) was sufficiently soft, and showed superior shape conformability that makes it an excellent choice as a subdural electrode used on the brain surface. In vivo measurement proved that the salt bridge electrode makes close contact to the exposed porcine brain and can record brain wave signals of frequencies 1 ~ 15 Hz. In addition, the high transparency of the electrode provided a clear view of the brain surface that would assist the effective surgical operation and optogenetic research.






Similar content being viewed by others
References
H. Abe, H. Yabu, R. Kunikata, A. Suda, M. Matsudaira, T. Matsue, Redox cycling-based electrochemical CMOS imaging sensor for real time and selective imaging of redox analyte. Sensor. Actuat. B-Chem. 304, 127245 (2020)
S. Budday, R. Nay, R. de Rooji, P. Steinmann, T. Wyrobek, T.C. Oyaert, E. Kuhl, Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 46, 318–330 (2015)
Y. Chen, Y. Zhang, Z. Liang, Y. Cao, Z. Han, X. Feng, Flexible inorganic bioelectronics. Npj Flex. Electron. 4(1), 2 (2020)
S. Dong, W. Chen, X. Wang, S. Zhang, K. Xu, X. Zheng, Flexible ECoG electrode for implantation and neural signal recording applications. Vacuum 140, 96–100 (2017)
K. Feron, R. Lim, C. Sherwood, A. Keynes, A. Brichta, P.C. Dastoor, Organic bioelectronics: Materials and biocompatibility. Int. J. Mol. Sci. 19(8), 2382 (2018)
T. Fujii, PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 61–62, 907–914 (2002)
Y. Gao, K. Wu, Z. Suo, Photodetachable Adhesion. Adv. Mater. 31(6), 1806948 (2018)
C. Heo, H. Park, Y.-T. Kim, E. Baeg, Y.H. Kim, S.-G. Kim, M. Suh, A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 6(1), 27818 (2016)
Y. Hou, C. Chen, K. Liu, Y. Tu, L. Zhang, Y. Li, Preparation of PVA hydrogel with high-transparence and investigations of its transparent mechanism. RSC Adv. 5(31), 24023–24030 (2015)
A.M. Hubbard, W. Cui, Y. Huang, R. Takahashi, M.D. Dickey, J. Genzer, D.R. King, J.P. Gong, Hydrogel/elastomer laminates bonded via fabric interphases for stimuli-responsive actuators. Matter 1(3), 674–689 (2019)
D.S. Kalinina, D.S. Vasilev, A.B. Volnova, N.N. Nalivaeva, I.A. Zhuravin, Age-dependent Electrocorticogram dynamics and epileptogenic responsiveness in rats subjected to prenatal hypoxia. Dev. Neurosci. 41(1–2), 56–66 (2019)
E. Kamio, T. Yasui, Y. Iida, J.P. Gong, H. Matsuyama, Inorganic/Organic Double-Network Gels Containing Ionic Liquids. Adv. Mater. 29(47), 1704118 (2017)
D. Khodagholy, J.N. Gelinas, T. Thesen, W. Doyle, O. Devinsky, G.G. Malliaras, G. Buzsáki, NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 18(2), 310–315 (2015)
W.-S. Kim, I.-H. Yun, J.-J. Lee, H.-T. Jung, Evaluation of mechanical interlock effect on adhesion strength of polymer–metal interfaces using micro-patterned surface topography. Int. J. Adhes. Adhes. 30(6), 408–417 (2010)
H. Lee, B.P. Lee, P.B. Messersmith, A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448(7151), 338–341 (2007)
J.H. Lee, H. Kim, J.H. Kim, S.-H. Lee, Soft implantable microelectrodes for future medicine: Prosthetics, neural signal recording and neuromodulation. Lab Chip 16(6), 959–976 (2016)
R.P. Lesser, N.E. Crone, W.R.S. Webber, Subdural electrodes. Clin. Neurophysiol. 121(9), 1376–1392 (2010)
Y. Liu, J. Liu, S. Chen, T. Lei, Y. Kim, S. Niu, H. Wang, X. Wang, A.M. Foudeh, J.B.-H. Tok, Z. Bao, Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat Biomed Eng 3, 58–68 (2019)
X. Liu, J. Liu, S. Lin, and X. Zhao, Hydrogel machines. Mater. Today (2020)
R.d.A. Nogueira, D.T. Pessoa, E.L.A. da Silva, E.V.L. Costa, Can a hypercholesterolemic diet change the basal brain electrical activity and during status epilepticus in rats? Metab. Brain Dis. 34(1), 71–77 (2019)
M. Ochoa, P. Wei, A.J. Wolley, K.J. Otto, B. Ziaie, A hybrid PDMS-Parylene subdural multi-electrode array. Biomed. Microdevices 15(3), 437–443 (2013)
S. Oribe, S. Yoshida, S. Kusama, S. Osawa, A. Nakagawa, M. Iwasaki, T. Tominaga, M. Nishizawa, Hydrogel-Based Organic Subdural Electrode with High Conformability to Brain Surface. Sci. Rep. 9(1), 13379 (2019)
D.W. Park, A.A. Schendel, S. Mikael, S.K. Brodnick, T.J. Richner, J.P. Ness, M.R. Hayat, F. Atry, S.T. Frye, R. Pashaie, S. Thongpang, Z. Ma, J.C. Williams, Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5(1), 1–11 (2014)
T.M. Reidy, D. Luo, P. Rana, B. Huegel, X. Cheng, Transparency of PDMS based microfluidic devices under temperature gradients. J. Micromechanics Microengineering 29(1), 015014 (2019)
Y. Ren, J. Guo, Z. Liu, Z. Sun, Y. Wu, L. Liu, F. Yan, Ionic liquid–based click-ionogels. Sci. Adv. 5(8), eaax0648 (2019)
L. Ronan, N. Voets, C. Rua, A. Alexander-Bloch, M. Hough, C. Mackay, T.J. Crow, A. James, J.N. Giedd, P.C. Fletcher, Differential tangential expansion as a mechanism for cortical gyrification. Cereb. Cortex 8, 2219–2228 (2014)
B. Rubehn, C. Bosman, R. Oostenveld, P. Fries, T. Stieglitz, A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 6(3), 036003 (2009)
T. Sakaguchi, S. Nagano, M. Hara, S.-H. Hyon, M. Patel, K. Matsumura, Facile preparation of transparent poly(vinyl alcohol) hydrogels with uniform microcrystalline structure by hot-pressing without using organic solvents. Polym. J. 49(7), 535–542 (2017)
A. Shah, S. Mittal, Invasive electroencephalography monitoring: Indications and presurgical planning. Ann. Indian Acad. Neurol. 17, 89(5) (2014)
E. Song, J. Li, S.M. Won, W. Bai, J.A. Rogers, Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 19, 590–603 (2020)
A. Suarez-Perez, G. Gabriel, B. Rebollo, X. Illa, A. Guimerà-Brunet, J. Hernández-Ferrer, M.T. Martínez, R. Villa, M.V. Sanchez-Vives, Quantification of signal-to-noise ratio in cerebral cortex recordings using flexible MEAs with co-localized platinum black, carbon nanotubes, and gold electrodes. Front. Neurosci. 12, 862 (2018)
C. Sung, W. Jeon, K.S. Nam, Y. Kim, H. Butt, S. Park, Multimaterial and multifunctional neural interfaces: From surface-type and implantable electrodes to fiber-based devices. J. Mater. Chem. B 8(31), 6624–6666 (2020)
R. Takahashi, K. Shimano, H. Okazaki, T. Kurokawa, T. Nakajima, T. Nonoyama, D.R. King, J.P. Gong, Tough Particle-Based Double Network Hydrogels for Functional Solid Surface Coatings. Adv. Mater. Interfaces 5(23), 1801018 (2018)
K. Tian, J. Bae, Z. Suo, J.J. Vlassak, Adhesion between hydrophobic elastomer and hydrogel through hydrophilic modification and interfacial segregation. ACS Appl. Mater. Interfaces 10(49), 43252–43261 (2018)
J. Yang, R. Bai, B. Chen, Z. Suo, Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 30(2), 1901693 (2020)
H. Yi, S.H. Lee, M. Seong, M.K. Kwak, H.E. Jeong, Bioinspired reversible hydrogel adhesives for wet and underwater surfaces. J. Mater. Chem. B 6(48), 8064–8070 (2018)
S. Yoshida, K. Sumomozawa, K. Nagamine, M. Nishizawa, Hydrogel Microchambers Integrated with Organic Electrodes for Efficient Electrical Stimulation of Human iPSC-Derived Cardiomyocytes. Macromol. Biosci. 19(6), 1900060 (2019)
H. Yuk, T. Zhang, S. Lin, G.A. Parada, X. Zhao, Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 15(2), 190–196 (2016)
H. Yuk, B. Lu, X. Zhao, Hydrogel bioelectronics. Chem. Soc. Rev. 48(6), 1642–1667 (2019)
W. Zhang, L. Chen, J. Zhang, Z. Huang, Design and optimization of carbon nanotube/polymer actuator by using finite element analysis. Chinese Phys. B 26(4), 048801 (2017)
S. Zhang, F. Wang, H. Peng, J. Yan, G. Pan, Flexible highly sensitive pressure sensor based on ionic liquid gel film. ACS Omega 3(3), 3014–3021 (2018)
S. Zhao, P. Tseng, J. Grasman, Y. Wang, W. Li, B. Napier, B. Yavuz, Y. Chen, L. Howell, J. Rincon, F.G. Omenetto, D.L. Kaplan, Programmable Hydrogel Ionic Circuits for Biologically Matched Electronic Interfaces. Adv. Mater. 30(25), e1800598 (2018)
G. Zhao, Y. Zhang, N. Shi, Z. Liu, X. Zhang, M. Wu, C. Pan, H. Liu, L. Li, Z.L. Wang, Transparent and stretchable Triboelectric Nanogenerator for self-powered tactile sensing. Nano Energy 59, 302–310 (2019)
Acknowledgements
This work was partly supported by Tohoku University Frontier Research program (FRiD), AMED-Medical Device Development (20331061) from Japan Agency for Medical Research and Development (AMED), and by Grant-in-Aids for Scientific Research A (18H04157) (18H04158), Scientific Research B (19H03755), Scientific Research C (19 K08090) (18 K08932) (18 K08960) (18 K08561) (17 K11373) (16 K10780) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishimura, A., Suwabe, R., Ogihara, Y. et al. Totally transparent hydrogel-based subdural electrode with patterned salt bridge. Biomed Microdevices 22, 57 (2020). https://doi.org/10.1007/s10544-020-00517-0
Published:
DOI: https://doi.org/10.1007/s10544-020-00517-0