Introduction
Tetrahedrite-group minerals are the most common sulfosalts in different kinds of hydrothermal ore deposits. They form a complex isotypic series, with the formula M (2)A6M (1)(B4C2)X (3)D4S(1)Y12S(2)Z, characterised by homo- and heterovalent substitutions, representing an interesting link between mineralogy and ore geochemistry (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020).
Within the tetrahedrite group, five different series have been identified: tetrahedrite, tennantite, freibergite, hakite and giraudite series. Other unassigned members (e.g. rozhdestvenskayaite and goldfieldite) belong to this group. Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020), on the basis of literature data, defined thirty-two potential end-member compositions within the tetrahedrite group; among them eleven can be considered as valid species, whereas the remaining phases have to be approved officially by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC).
The most common mineral species within the tetrahedrite group belong to the tetrahedrite and tennantite series, characterised by B = Cu, D = Sb, Y = S, and B = Cu, D = As, Y = S, respectively. Within these two series, the only approved species have C = Zn or Fe, i.e. tetrahedrite-(Fe), tetrahedrite-(Zn), tennantite-(Fe), and tennantite-(Zn). However, a critical examination of available literature reveals that other tetrahedrite- and tennantite-series minerals, characterised by different C constituents, have been reported. Among them, tetrahedrite where C = Hg, i.e. Hg-rich tetrahedrite, is widely known, sometimes referred to with the term ‘schwazite’ (e.g. Arlt and Diamond, Reference Arlt and Diamond1998). The more complete description of ‘schwazite’ is probably that reported by Karanović et al. (Reference Karanović, Cvetković, Poleti, Balić-Žunić and Makovicky2003) using a specimen from Dragodol, Donja Trešnjica district, Serbia. However, their chemical data, collected using energy-dispersive spectrometry (EDS) mode, were of low quality, contrasting with the goodness of their crystallographic data. Foit and Hughes (Reference Foit and Hughes2004) studied the structural variations of a solid solution between tetrahedrite-(Zn) and tetrahedrite-(Hg) from the Spring Creek Claims, Harney County, Oregon, USA; the sample richest in Hg has chemical composition Cu6(Cu4.08Hg1.15Zn0.67Fe0.03Co0.01)Σ5.94(Sb2.64As1.36)Σ4.00S13. In either case, type material could not be located.
Tetrahedrite-(Hg) was identified also at the Buca della Vena mine, Apuan Alps, Tuscany, Italy, and its occurrence and crystal-chemistry has been recently characterised. In addition to two well-characterised occurrences from the Jedová hora deposit, Czech Republic, and the Rožňava ore field, Slovakia, these new data allowed the submission of a formal proposal to the IMA–CNMNC, in order to give an official certificate of birth to tetrahedrite-(Hg). The mineral and its name have been approved (IMA2019-003, Biagioni et al., Reference Biagioni, Sejkora, Musetti, Velebil and Pasero2019). Holotype material is deposited in the mineralogical collections of the Museo di Storia Naturale, Università di Pisa, Via Roma 79, Calci (Pisa), Italy, catalogue number 19895. Both cotype samples are deposited in the mineralogical collection of the Department of Mineralogy and Petrology, National Museum, Prague, Czech Republic, catalogue numbers: P1N 9961 (Jedová hora) and P1N 33538 (Rožňava).
In this paper, the description of tetrahedrite-(Hg) is given, along with a brief history of previous studies on this mineral species.
Occurrence and physical properties
Tetrahedrite-(Hg) was found in the pyrite ± baryte ± iron-oxide ore deposit of Buca della Vena mine (43°59’55’’N, 10°18’37’’E), Stazzema, Apuan Alps, Lucca Province, Tuscany, Italy. The ore bodies are located at the contact between a metavolcanic–metasiliciclastic sequence belonging to the Palaeozoic basement of the Apuane Unit and the Triassic metadolostone of the Grezzoni Formation (Benvenuti et al., Reference Benvenuti, Lattanzi, Tanelli and Cortecci1986). Their textural and mineralogical features are affected by the greenschist-facies metamorphism of Alpine age that characterises all the deposits of the southern Apuan Alps (e.g. D'Orazio et al., Reference D'Orazio, Biagioni, Dini and Vezzoni2017). Ore bodies have a simple primary mineralogy, basically formed by baryte, magnetite, hematite and pyrite. However, the Buca della Vena mine is renowned among mineralogists and mineral collectors for several very rare mineral species that have been described from here since the end of the 1970s. Among them, twelve have this mine as their type locality: allanite-(La), apuanite, bohuslavite, dessauite-(Y), mapiquiroite, marrucciite, oxycalcioroméite, pellouxite, pillaite, rouxelite, scainiite and versiliaite. Tetrahedrite-(Hg) is the thirteenth new mineral species found at this locality.
Tetrahedrite-(Hg) was identified in samples collected in an exploitation void known, among mineral collectors, as the Sala del Castello. Here, a metre-sized metadolostone lens occurs embedded in microcrystalline baryte + iron-oxides and it is cut by several sets of veins and fractures. Tetrahedrite-(Hg) occurs as anhedral grains or equant crystals, up to 0.2 mm in size (Fig. 1a), black in colour and with a black streak. The lustre is metallic. The Mohs hardness may be close to 3½–4, in agreement with other members of the tetrahedrite group and with the sample of Hg-rich tetrahedrite from Moschellandsberg (Germany) that has a Vickers hardness of 236–277 kg/mm2 (Criddle and Stanley, Reference Criddle and Stanley1993). Tetrahedrite-(Hg) is brittle, with an indistinct cleavage and a conchoidal fracture. Density was not measured, owing to the small amount of available material; on the basis of the empirical formula and the single-crystal unit-cell parameters, the calculated density is 5.326 g/cm3.
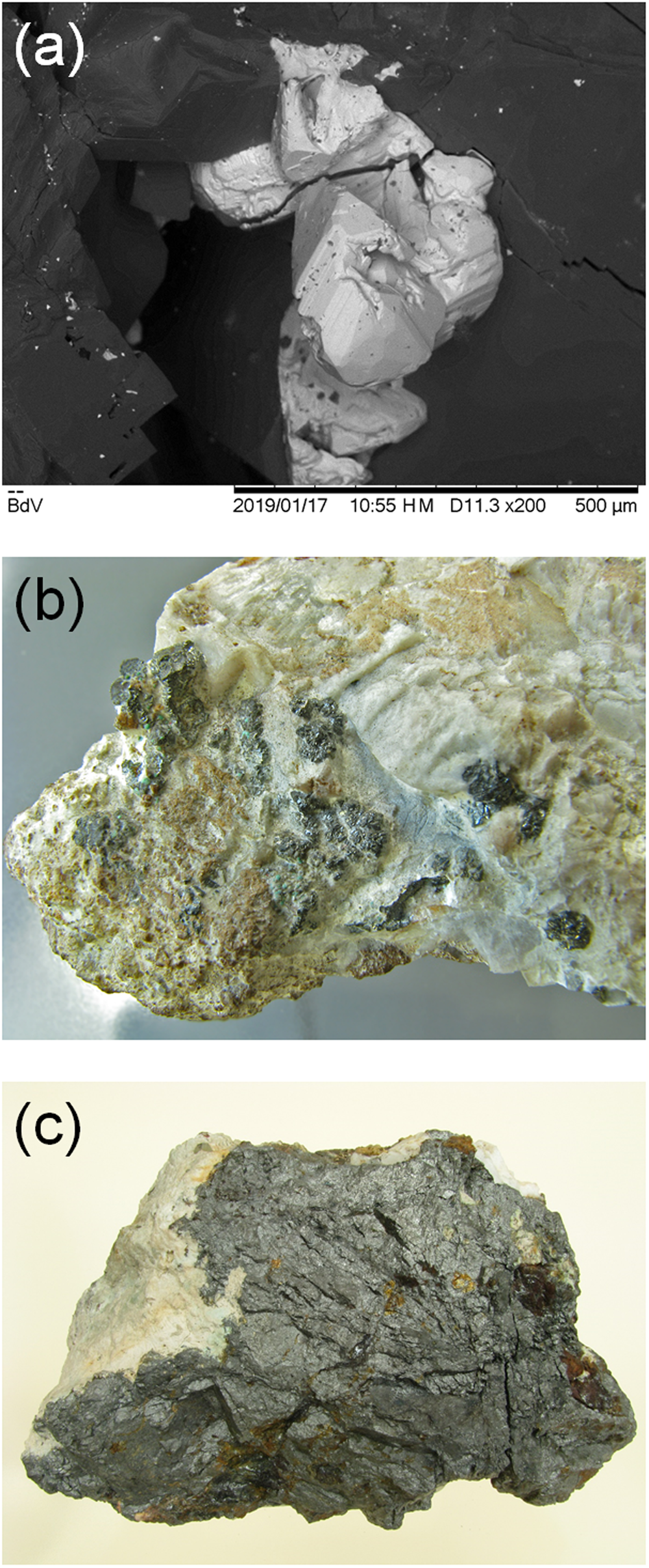
Fig. 1. Type material for tetrahedrite-(Hg). (a) Tetrahedral crystals up to 0.2 mm, with dolomite. Buca della Vena mine, Apuan Alps, Tuscany, Italy (sample no. 19895). (b) Aggregates up to 4 mm with baryte. Field of view: 5 cm. Jedová hora deposit, Czech Republic (sample no. P1N 9961). (c) Massive aggregate with quartz. Size: 11 cm × 6 cm × 7 cm. Rožňava ore field, Slovakia (sample no. P1N 33538).
In reflected light, tetrahedrite-(Hg) is isotropic. It is greyish-white, with creamy tints. Internal reflections were not observed. Reflectance values, measured in air using a spectrophotometer MSP400 Tidas at Leica microscope, with a 100× objective, are given in Table 1 and shown in Fig. 2.

Fig. 2. Reflectance curves for tetrahedrite-(Hg) in air. For comparison, the reflectance curves of type material from Buca della Vena, cotype samples from Jedová hora and Rožňava, and literature data are shown.
Table 1. Reflectance data for tetrahedrite-(Hg) from Buca della Vena, Jedová hora, and Rožňava. Literature data are reported for comparison.

The values required by the Commission on Ore Mineralogy for the type material from Buca della Vena mine are shown in bold.
Data source: [1] This work; [2] Criddle and Stanley (1993); [3] Karanović et al. (2003).
Tetrahedrite-(Hg) is associated with cinnabar, as small μm-sized equant deep red crystals, and chalcostibite, as striated tabular prismatic crystals, up to 0.2 mm long, in vugs of dolomite veins. It is worth noting that other Hg minerals have been identified at the same occurrence: coloradoite (Dini and Orlandi, Reference Dini and Orlandi1995), rouxelite (Orlandi et al., Reference Orlandi, Meerschaut, Moëlo, Palvadeau and Léone2005), marrucciite (Orlandi et al., Reference Orlandi, Moëlo, Campostrini and Meerschaut2007), tiemannite and metacinnabar (Biagioni and Orlandi, Reference Biagioni and Orlandi2009). The crystallisation of tetrahedrite-(Hg) is related to the circulation of hydrothermal fluids during the Tertiary Alpine tectono-metamorphic events.
During this study, in addition to the sample from the Buca della Vena mine, two other samples were examined. Tetrahedrite-(Hg) was identified from the Jedová hora deposit (49°47′31″N, 13°53′13″E), Hořovice, Central Bohemian Region, Czech Republic (Fig. 1b), and from the Rožňava ore field (48°40′37″N, 20°32′29″E), Košice Region, Slovakia (Fig. 1c). The sample from the Jedová hora deposit (vertical fissures with hydrothermal mineralisation – quartz, siderite, dolomite, pyrite, baryte, cinnabar and tetrahedrite-(Hg) – crosscutting two stratiform bodies of iron ores – siderite and hematite) is represented by groups of imperfect crystals of tetrahedrite-(Hg) up to 4 mm in size on baryte, in association with chalcopyrite (sample 8 cm × 4.5 cm × 2.5 cm). The sample from the Rožňava ore field probably came from the upper part of the Mária vein (hydrothermal siderite–quartz vein with chalcopyrite, pyrite and tetrahedrite with variable Hg contents) and it is represented by massive tetrahedrite-(Hg) aggregates up to 10 cm in association with quartz (sample 11 × 6 × 7 cm). It is worth noting that tetrahedrite-group minerals from the Rožňava ore field are typically Hg-poor. However, the sample studied came to the museum in 1941 from the well-known and careful scientist Radim Nováček (1905–1942), who investigated this sample and published his results (Nováček, Reference Nováček1942) (see Fig. S1, available as Supplementary Material). Optical properties of both samples are similar to those reported for the Italian type material and the reflectance values are given in Table 1 and shown in Fig. 2.
Chemical data
Quantitative chemical analyses were carried out using a Cameca SX 100 electron microprobe at the National Museum of Prague, Czech Republic. Experimental conditions were: wavelength-dispersive spectroscopy mode, accelerating voltage 25 kV, beam current 20 nA and beam diameter 1 μm. Standards (element, emission line) were: pyrite (FeKα), chalcopyrite (CuKα and SKα), ZnS (ZnKα), NiAs (AsLα), Ag metal (AgLα), Sb2S3 (SbLα), PbSe (SeLα), HgTe (HgMα) and Bi2Se3 (BiMα). Matrix correction by PAP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data. Chemical data for tetrahedrite-(Hg) from Buca della Vena mine, along with samples from Jedová hora and Slovakia, are given in Table 2.
Table 2. Chemical data for tetrahedrite-(Hg) from Buca della Vena, Jedová hora and Rožňava.

Notes: Ev(%) = [Σ(val+) −Σ(val−)] × 100 / Σ(val−); Fe assumed as Fe2+. e.s.d. = estimated standard deviation. ‘–’ below detection limit.
Empirical formulae of tetrahedrite-(Hg) samples: Buca della Vena: (Cu9.44Ag0.07)Σ9.51(Hg1.64Zn0.36Fe0.06)Σ2.06Sb4(S12.69Se0.01)Σ12.70, Jedová hora: Cu9.69(Hg1.75Fe0.25Zn0.06)Σ2.06(Sb3.94As0.06)S12.87 and Rožňava: (Cu9.76Ag0.04)Σ9.80(Hg1.83Fe0.15Zn0.10)Σ2.08(Sb3.17As0.58Bi0.25)S13.01. The partitioning of Cu and Ag between A and B constituents gives the formulae: Buca della Vena: (Cu5.50Ag0.07)Σ5.57(Cu3.94Hg1.64Zn0.36Fe0.06)Σ6.00Sb4(S12.69Se0.01)Σ12.70, Jedová hora: Cu5.75(Cu3.94Hg1.75Fe0.25Zn0.06)Σ6.00(Sb3.94As0.06)S12.87, and Rožňava: (Cu5.84Ag0.04)Σ5.88(Cu3.92Hg1.83Fe0.15Zn0.10)Σ6.00(Sb3.17As0.58Bi0.25)S13.01.
The empirical formulae of the samples of tetrahedrite-(Hg), based on (As+Sb+Bi) = 4 atoms per formula unit (apfu), are given in the footnote of Table 2, along with the formulae written according to the partitioning of Cu and Ag between A and B constituents.
The specimens studied show a deficit of (Cu + Ag) (up to 0.43 apfu in the sample from Buca della Vena mine), and two samples also a slight S deficit (up to 0.30 apfu). The deficit of (Cu + Ag) is discussed below.
The end-member formula of tetrahedrite-(Hg) is Cu6(Cu4Hg2)Sb4S13 (Z = 2), corresponding to (in wt.%): Cu 32.75, Hg 20.67, Sb 25.10, S 21.48, total 100.00.
X-ray crystallography
Powder X-ray diffraction pattern of tetrahedrite-(Hg) from the Buca della Vena mine (Table 3) was collected using a 114.6 mm Gandolfi camera and Ni-filtered CuKα radiation (Department of Earth Sciences, University of Pisa, Italy). Unit-cell parameters were refined on the basis of 18 unequivocally indexed reflections using the software UnitCell (Holland and Redfern, Reference Holland and Redfern1997): a = 10.5072(6) Å and V = 1160.0(2) Å3. Powder X-ray diffraction data of both cotype samples were recorded using a Bruker D8 Advance diffractometer equipped with solid-state LynxEye detector and secondary monochromator producing CuKα radiation housed at the Department of Mineralogy and Petrology, National Museum, Prague, Czech Republic. The instrument was operating at 40 kV and 40 mA. In order to minimise the background, the powder samples were placed on the surface of a flat silicon wafer. The powder patterns were collected in Bragg–Brentano geometry in the range 3–75°2θ, step 0.01° and counting time of 20 s per step (total duration of experiment was ca. 3 days). The positions and intensities of diffractions were found and refined using the Pearson VII profile-shape function of the ZDS program package (Ondruš, Reference Ondruš1993) and the unit-cell parameters were refined by the least-squares program of Burnham (Reference Burnham1962). Unit-cell parameters, refined from powder X-ray diffraction data given in Table 3, are a = 10.4939(1) Å and V = 1155.61(5) Å3 for the sample from Jedová hora, Czech Republic and a = 10.4725(1) Å and V = 1148.55(6) Å3 for the sample from Rožňava, Slovakia.
Table 3. Observed and calculated powder X-ray diffraction data for tetrahedrite-(Hg) from Buca della Vena along with Jedová hora and Rožňava for comparison.

Notes: Intensity and d hkl were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model given in Table 5. Only reflections with I calc > 2 are listed, if not observed. The eight strongest reflections are given in bold. Observed intensities were visually estimated (vs = very strong; m = medium; mw = medium-weak; w = weak; vw = very weak).
For the refinement of the crystal structure, X-ray diffraction intensity data were collected on tetrahedrite-(Hg) from Buca della Vena mine using a Bruker Smart Breeze diffractometer (50 kV and 30 mA) equipped with a Photon II CCD detector and graphite-monochromatised MoKα radiation. The detector-to-crystal distance was set at 50 mm. Data were collected using ω scan mode in 0.5° slices, with an exposure time of 10 s per frame, and they were corrected for Lorentz and polarisation factors as well as for absorption using the software package Apex3 (Bruker AXS Inc., 2016). The refined unit-cell parameter is a = 10.5057(8) Å and V = 1159.5(3) Å3; and space group I ![]() $\bar{4}$3m. The crystal structure of tetrahedrite-(Hg) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of tetrahedrite given by Johnson and Burnham (Reference Johnson and Burnham1985). The occurrence of racemic twins was modelled, with a ratio between the two components of 94(3):6(3). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used: Cu at M(2); Cu vs. Hg at M(1); Sb at X(3); and S at S(1) and S(2) sites. The anisotropic structural model converged to R 1 = 0.0190 for 335 reflections with F o > 4σ(F o) and 20 refined parameters. Details of data collection and refinement are given in Table 4. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 5. Table 6 reports selected bond distances. Finally, Table 7 gives the bond-valence calculations obtained using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
$\bar{4}$3m. The crystal structure of tetrahedrite-(Hg) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the structural model of tetrahedrite given by Johnson and Burnham (Reference Johnson and Burnham1985). The occurrence of racemic twins was modelled, with a ratio between the two components of 94(3):6(3). The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were used: Cu at M(2); Cu vs. Hg at M(1); Sb at X(3); and S at S(1) and S(2) sites. The anisotropic structural model converged to R 1 = 0.0190 for 335 reflections with F o > 4σ(F o) and 20 refined parameters. Details of data collection and refinement are given in Table 4. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 5. Table 6 reports selected bond distances. Finally, Table 7 gives the bond-valence calculations obtained using the bond-valence parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991). The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Summary of crystal data and parameters describing data collection and refinement for tetrahedrite-(Hg).

Table 5. Sites, Wyckoff positions, site occupancy factors (s.o.f.), fractional atom coordinates and equivalent isotropic displacement parameters (Å2) for tetrahedrite-(Hg).

Table 6. Selected bond distances (Å) for tetrahedrite-(Hg).

Table 7. Bond-valence sums (in valence units) for tetrahedrite-(Hg).

Note: left and right superscripts indicate the number of equivalent bonds involving cations and anions, respectively.
Results and discussion
Crystal structure description
The crystal structure of tetrahedrite-(Hg) agrees with the general features of the members of the tetrahedrite isotypic group. It can be described as a collapsed sodalite-like framework of corner-sharing M(1)-centred tetrahedra, with cages hosting S(2)-centred M(2)-octahedra and four X(3)S(1)3 trigonal pyramids (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1988).
In the sample studied, the triangularly coordinated M(2) site has an average bond distance of 2.262 Å. Refined site scattering, as well as the calculated bond-valence sum (BVS) of 1.02 valence units (vu), agree with the full occupancy at this position by Cu. Electron microprobe analysis suggest the occurrence of a minor vacancy (0.43 apfu). This may be due to some analytical problems, such as the migration of Cu under the electron beam. However, this phenomenon was reported only for Cu-excess tetrahedrites, both natural and synthetic (e.g. Lind and Makovicky, Reference Lind and Makovicky1982). If a minor vacancy is real, this may be coupled with the partial oxidation of Cu+ to Cu2+ at the M(1) site, giving an excess of divalent metals, as observed in pošepnýite (Škácha et al., Reference Škácha, Sejkora, Plášil and Makovicky2020). It is not unlikely that such a minor amount of vacancy could be overlooked, because the overestimated site scattering (29 electrons vs. 27.1 electrons, as calculated from chemical data) would be partially masked by the relatively large U eq value due to the positional disorder affecting the M(2) site, that is actually split into two flat pyramidal sub-sites, as reported previously by other authors (e.g. Andreasen et al., Reference Andreasen, Makovicky, Lebech and Karup-Møller2008; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). The splitting of this position did not improve significantly the crystal structure refinement of tetrahedrite-(Hg) and consequently an unsplit model was preferred.
The tetrahedrally coordinated M(1) site has an average bond distance of 2.391 Å. Assuming the site population (Cu0.66Hg0.28Zn0.05Fe0.01), based on chemical data, the theoretical bond distance at M(1) is 2.393 Å, as obtained using the atomic radii proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1988). The refined site scattering (44.3 electrons per site) is in accord with the site scattering calculated from the site population (43.0 electrons). Bond-valence sums show an overbonding at M(1). This is a usual feature agreeing with previous studies on sulfosalts having tetrahedrally coordinated Hg (e.g. arsiccioite, AgHg2TlAs2S6 – Biagioni et al., Reference Biagioni, Bonaccorsi, Moëlo, Orlandi, Bindi, D'Orazio and Vezzoni2014c) and it is possibly due to the inaccuracy of the bond parameters for the Hg–S pair or to a small shift of the S positions when the M(1) site is occupied by Hg.
The X(3) site has an average bond distance of 2.447 Å, fully consistent with a pure Sb occupancy, as indicated by the BVS of 3.03 vu.
The S(1) site is four-fold coordinated, being bonded to two M(1), one M(2) and one X(3). Its BVS is 2.16 vu. S(2) is octahedrally coordinated by atoms hosted at M(2) sites, with BVS of 2.16 vu. No hints of vacancies at S(2) were observed during the structure refinement.
Relationships between geometrical features and chemistry
Several authors proposed some relationships between chemistry and geometrical features of tetrahedrite-group minerals (e.g. Charlat and Lévy, Reference Charlat and Lévy1975; Mozgova and Tsepin, Reference Mozgova and Tsepin1983; Johnson et al., Reference Johnson, Craig and Rimstidt1987; Makovicky and Karup-Møller, Reference Makovicky and Karup-Møller1994). Johnson et al. (Reference Johnson, Craig and Rimstidt1987) proposed a relationship between the unit-cell parameter and chemistry:
where Cu* = 2.0 – (Fe + Zn + Hg + Cd) and the coefficient of the Hg term is corrected according to Di Benedetto et al. (Reference Di Benedetto, Bernardini, Borrini, Emiliani, Cipriani, Danti, Caneschi, Gatteschi and Romanelli2002). As the sum of Fe, Zn and Hg is slightly larger than 2 apfu in all the three samples studied, the term Cu* was ignored. Applying such a relationship, the unit-cell parameters a = 10.493, 10.492 and 10.518 Å can be calculated for tetrahedrite-(Hg) from Buca della Vena, Jedová hora and Rožňava, respectively. These values have to be compared with the observed ones, i.e. a = 10.506 Å (Buca della Vena), 10.494 Å (Jedová hora) and 10.472 Å (Rožňava).
The three samples studied have different compositions of the tetrahedral M(1) site. Indeed, whereas among divalent constituents the main Hg substituent in the sample from Buca della Vena is Zn2+ [Zn/(Fe + Zn + Hg) = 0.17], with negligible Fe (less than 0.10 apfu), the samples from Jedová hora and Rožňava have Fe > Zn [Zn/(Fe + Zn + Hg) = 0.03 and 0.05, respectively]. For this reason, the relationship between the unit-cell parameter and the Zn and Hg content proposed by Karup-Møller and Makovicky (Reference Karup-Møller and Makovicky2004) can be applied to the sample from Buca della Vena: a (Å) = 0.0293 Zn (apfu) + 0.096 Hg (apfu) + 10.3245. The calculated value, 10.492 Å, is close to the value obtained through the relationship of Johnson et al. (Reference Johnson, Craig and Rimstidt1987), and slightly smaller than the observed value (10.506 Å).
Taking into account the crystal radii proposed by Johnson et al. (Reference Johnson, Craig and Rimstidt1988) and the site population given above, the M(1)–S(1) bond distance in the sample from Buca della Vena should be 2.393 Å, in perfect agreement with the observed value, 2.393 Å. Moreover, Foit and Hughes (Reference Foit and Hughes2004) proposed a linear relationship between Hg content and the M(1)–S(1) bond distance. According to their relationship, the M(1)–S(1) distance in tetrahedrite-(Hg) from Buca della Vena mine should be 2.385 Å, slightly shorter than the observed value, probably as a consequence of the occurrence of minor Zn and Fe replacing Cu. The observed value is comparable with the M(1)–S(1) distance reported by Karanović et al. (Reference Karanović, Cvetković, Poleti, Balić-Žunić and Makovicky2003) in tetrahedrite-(Hg) from Dragodol, Serbia, 2.382 Å, and in the only Hg-dominant sample studied by Foit and Hughes (Reference Foit and Hughes2004), 2.371 Å. These values are definitely longer than those reported in tetrahedrite-(Fe) and tetrahedrite-(Zn) (e.g. 2.342 Å – Wuensch, Reference Wuensch1964).
Previous findings of tetrahedrite-(Hg): a short review
The occurrence of Hg-rich tetrahedrite, in some cases corresponding to tetrahedrite-(Hg), has been known since the 19th Century (e.g. Breithaupt, 1816 in Palache et al., Reference Palache, Berman and Frondel1951; Kersten, Reference Kersten1843; Rath, Reference Rath1855). Mercury-rich tetrahedrite is widely known with the term ‘schwazite’ (sometimes misspelled as ‘schwatzite’), from the locality Schwaz, Tyrol, Austria. Indeed, Weidenbusch (Reference Weidenbusch1849) reported the analysis of a tetrahedrite-group mineral from this locality having 15.9 wt.% Hg and corresponding to the formula Cu9.9Hg1.4Fe0.7Zn0.4Sb3.2S13. The name ‘schwazite’ was first introduced by Kenngott (Reference Kenngott1853). Arlt and Diamond (Reference Arlt and Diamond1998) reviewed occurrences of ‘schwazite’, and showed that none of the authors involved in the study of ‘schwazite’ from Schwaz, following Weidenbusch (Reference Weidenbusch1849), found Hg contents higher than 5.4 wt.%. Moreover, they reported new chemical data, finding up to 9.4 wt.% Hg, corresponding to 0.82 Hg apfu. Therefore, at Schwaz there are no samples having Hg as the dominant divalent cation (Arlt and Diamond, Reference Arlt and Diamond1998), discrediting the name ‘schwazite’ (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020).
Several other occurrences of Hg-rich tetrahedrite are known. Mozgova et al. (Reference Mozgova, Tsepin, Ozerova, Bortnikov and Tronieva1979) reviewed the occurrences of Hg-bearing tetrahedrites, identifying four possible kinds of occurrence. They listed (1) cinnabar deposits, (2) Hg–Sb deposits, (3) Hg tetrahedrite–tennantite deposits, and (4) low-Hg tetrahedrites in Sb–W, Pb–Ag and Au deposits. Moreover, Mozgova and Tsepin (Reference Mozgova and Tsepin1983) reported a dozen deposits where chemical analyses of tetrahedrite- and tennantite-series minerals gave Hg >18 wt.% (up to 21.8 wt.%) and (Fe + Zn) are <1 wt.%. However, not all of the Hg-bearing tetrahedrite samples reported in the literature can be classified as tetrahedrite-(Hg). For instance, Atanasov (Reference Atanasov1975) reported electron-microprobe and powder X-ray diffraction data of a sample from the Chiprovtsi Pb–Ag deposit, Western Stara–Planina mountains, Bulgaria. The average of three spot analyses gives the chemical formula Cu6.88Ag2.96(Hg1.83Zn0.17)Σ2.00(Sb3.36As0.71)Σ4.07S13.09. It is worth noting that if Cu is partitioned between M(1) and M(2), the possible dominance of Ag at M(2) results, i.e. (Ag2.96Cu2.88)Σ5.84. The classification of this sample, without further data, is uncertain, being classifiable either as an Ag-rich variety of tetrahedrite-(Hg) or as argentotetrahedrite-(Hg). Some true tetrahedrite-(Hg) can be found in the literature. Criddle and Stanley (Reference Criddle and Stanley1993) reported optical data for a sample of tetrahedrite-(Hg) from Moschellandsberg, Rhineland-Palatinate, Germany, having the chemical composition Cu9.97(Hg1.73Fe0.17)Σ1.90Sb4.12S13.64. Foit and Ulbricht (Reference Foit and Ulbricht2001) recorded up to 2.02 Hg apfu in samples from the epithermal deposits of the Steens and Pueblo mountains, Harney County, Oregon, USA, whereas Grammatikopoulos et al. (Reference Grammatikopoulos, Roth and Valeyev2005) measured up to 1.97 Hg apfu in Ag-bearing samples from the Eskay Creek deposit, British Columbia, Canada. Velebil (Reference Velebil2014) described the occurrence of tetrahedrite-(Hg) from some Czech and Slovak ore deposits, i.e. the Jedová hora deposit (1.46–1.73 Hg apfu) from the Czech Republic, and Rudňany (1.47–1.79 Hg apfu), Rožňava (1.65 Hg apfu) and Nižná Slaná (1.07–1.39 Hg apfu), all in the Slovak Republic.
The first crystallographic study was reported by Kalbskopf (Reference Kalbskopf1971) who proposed that Hg occurs at the tetrahedral position in Hg-rich tetrahedrite from Rošňava, ČSSR (Rožňava, now Slovak Republic). However, no quantitative chemical data and atomic coordinates were given. The crystal structure of tetrahedrite-(Hg) was solved by Kaplunnik et al. (Reference Kaplunnik, Pobedimskaya and Belov1980). Unfortunately, some very important details were misinterpreted or missing. Indeed, the refinement was performed using an incorrect 12 S apfu model, and the M(1)–S(1) distance (2.354 Å) agrees with the 1.02 Hg apfu content reported in their electron microprobe analysis, whereas a content of 1.6 Hg apfu on the basis of the a unit-cell parameter and the refined site occupancy was given. Karanović et al. (Reference Karanović, Cvetković, Poleti, Balić-Žunić and Makovicky2003) reported the crystal structure investigation of ‘schwazite’ from Dragodol, Donja Trešnjica district, Serbia. Electron microprobe data (EDS mode) gave the formula Cu9.28Hg1.64Zn0.76Fe0.02As0.17Sb4.19S13 [Ev(%) = +4.6]. This formula can be recalculated to Cu8.57Hg1.52Zn0.70Fe0.02Sb3.87As0.13S12.00, on the basis of (Sb + As) = 4 apfu. The poor quality of chemical data contrasts with the high quality of structural data [R 1 = 0.0148 for 165 reflections with F o > 4σ(F o)], confirming the occurrence of Hg at the tetrahedral M(1) site. Further structural data on Hg-rich tetrahedrite [actually a solid solution between tetrahedrite-(Zn) and tetrahedrite-(Hg)] were given by Foit and Hughes (Reference Foit and Hughes2004), who studied the structural variations in Hg-rich tetrahedrite from the Spring Creek Claims. The sample richest in Hg, corresponding to tetrahedrite-(Hg), has the composition Cu6(Cu4.08Hg1.15Zn0.67Fe0.03Co0.01)Σ5.94(Sb2.64As1.36)Σ4.00S13. Synthetic Hg-rich tetrahedrites (up to 2 Hg apfu) were studied by Johnson (Reference Johnson1991), who reported powder X-ray diffraction data and a unit-cell parameter a = 10.5071(1) Å, and by Karup-Møller and Makovicky (Reference Karup-Møller and Makovicky2003, Reference Karup-Møller and Makovicky2004), who examined Hg-rich tetrahedrite and the solid solution between Zn-rich and Hg-rich tetrahedrite.
Tetrahedrite-(Hg) and related phases
As described above, the crystal structure of tetrahedrite-(Hg) is formed by a three-dimensional framework composed by M(1)S(1)4 tetrahedra connected through corner sharing, delimiting cavities that can be described as truncated tetrahedra, the so-called Laves polyhedra. In these cavities, Z-centred M(2)6-octahedra are hosted, encircled by four XY 3 trigonal pyramids. The structural formula of tetrahedrite-(Hg) can thus be written as |Cu6Sb4S|[(Cu4Hg2)S12] (Z = 2). Tetrahedrite-(Hg) has isotypic relationships with the hypothetical end-member ‘tennantite-(Hg)’. This phase has actually been found in Nature (e.g. Mozgova et al., Reference Mozgova, Tsepin and Ozerova1978; Mozgova and Tsepin, Reference Mozgova and Tsepin1983). Mozgova et al. (Reference Mozgova, Tsepin and Ozerova1978) reported ‘tennantite-(Hg)’ from the ore deposit of Kulpolnei, Čukotka, Russia. The chemical formula, recalculated on the basis of three analyses (No. 2, 3–1, and 4 of table 1 in Mozgova et al., Reference Mozgova, Tsepin and Ozerova1978), corresponds to Cu5.68(Cu4.11Hg1.82Zn0.05Fe0.02)(As3.90Sb0.10)S13.07. ‘Tennantite-(Hg)’ has the unit-cell parameter a = 10.34 Å; the a parameter, shorter than that of tetrahedrite-(Hg), is in keeping with the As-to-Sb substitution and agrees with the value calculated applying the relation of Johnson et al. (Reference Johnson, Craig and Rimstidt1987), i.e. a = 10.35 Å.
Two other groups of phases share similar topologies with tetrahedrite-group minerals, with some important differences: the galkhaite and routhierite groups. The galkhaite group (‘galkhaites’) is currently formed by three different species: galkhaite, (Hg5Cu)CsAs4S12 (e.g. Biagioni et al., Reference Biagioni, Bindi and Zaccarini2014a), vorontsovite, (Hg5Cu)TlAs4S12, and ferrovorontsovite, (Fe5Cu)TlAs4S12 (Kasatkin et al., Reference Kasatkin, Nestola, Agakhanov, Škoda, Karpenko, Tsyganko and Plášil2018). These minerals are cubic, I ![]() $\bar{4}$3m, and have the same tetrahedral tetrahedrite-like framework, with (Hg,Cu)S4 or (Fe,Cu)S4 tetrahedra and AsS3 trigonal pyramids. The difference lies in the occupancy of the Laves polyhedra: whereas ZM(2)6 octahedra occur in tetrahedrite-group minerals (Fig. 3a), galkhaite-group compounds show Cs or Tl at the centre of the truncated tetrahedra, in twelve-fold coordination (Fig. 3b). Taking into account such a crystal-chemistry, the structural formula of galkhaite can be written as |CsAs4|[(Hg5Cu)S12].
$\bar{4}$3m, and have the same tetrahedral tetrahedrite-like framework, with (Hg,Cu)S4 or (Fe,Cu)S4 tetrahedra and AsS3 trigonal pyramids. The difference lies in the occupancy of the Laves polyhedra: whereas ZM(2)6 octahedra occur in tetrahedrite-group minerals (Fig. 3a), galkhaite-group compounds show Cs or Tl at the centre of the truncated tetrahedra, in twelve-fold coordination (Fig. 3b). Taking into account such a crystal-chemistry, the structural formula of galkhaite can be written as |CsAs4|[(Hg5Cu)S12].

Fig. 3. Structural fragments of the crystal structure of tetrahedrite group (a), galkhaite group (b), and routhierite group minerals (c), organised around the Laves polyhedra, showing their topological relationships. Polyhedra: grey = M(1) site in tetrahedrites, (Hg/Fe,Cu) site in galkhaites, and the M(2) site in routhierites; green = M(1) site in routhierites. Circles: green = M(2) site in tetrahedrites; orange = X(3) site in tetrahedrites; dark violet = As sites in galkhaites and routhierites; violet = (Cs/Tl) site in galkhaites; grey = Tl site in routhierites; yellow = S sites.
Mercury is an important constituent also in the routhierite-group minerals (hereafter routhierites). In these phases, the occurrence of two distinct kinds of tetrahedral sites, showing different occupancies, lowers the symmetry to I ![]() $\bar{4}$2m. Among the currently five known mineral species, two contain essential Hg: routhierite, CuHg2TlAs2S6 (Bindi, Reference Bindi2008; Biagioni et al., Reference Biagioni, Bonaccorsi, Moëlo and Orlandi2014b) and arsiccioite, AgHg2TlAs2S6 (Biagioni et al., Reference Biagioni, Bindi and Zaccarini2014a). Doubling the formula of routhierite, one obtains Cu2Hg4Tl2As4S12 (Z = 2), that can be written as |Tl2As4|[Cu2Hg4S12], discriminating the composition of the tetrahedral framework and the content of the distorted Laves polyhedron (Fig. 3c).
$\bar{4}$2m. Among the currently five known mineral species, two contain essential Hg: routhierite, CuHg2TlAs2S6 (Bindi, Reference Bindi2008; Biagioni et al., Reference Biagioni, Bonaccorsi, Moëlo and Orlandi2014b) and arsiccioite, AgHg2TlAs2S6 (Biagioni et al., Reference Biagioni, Bindi and Zaccarini2014a). Doubling the formula of routhierite, one obtains Cu2Hg4Tl2As4S12 (Z = 2), that can be written as |Tl2As4|[Cu2Hg4S12], discriminating the composition of the tetrahedral framework and the content of the distorted Laves polyhedron (Fig. 3c).
Passing from tetrahedrite-(Hg) to routhierites and then to galkhaites, the content of Hg in the tetrahedral framework increases, from 2 apfu to 5 apfu. The excess of positive charges is balanced by decreasing the charges hosted in the cavities, replacing six Cu+ atoms with one Tl2 dimer in routhierites and one large monovalent ion (Cs or Tl) in galkhaites. It seems likely that the tetrahedrite-like tetrahedral framework may be able to adapt to the variable ore geochemistry, allowing the incorporation of variable amounts of divalent elements (Hg, Zn and Fe) in tetrahedrite-group minerals as well as favouring the crystallisation of peculiar mineral phases like galkhaites and routhierites in environments characterised by the occurrence of large monovalent cations like Tl+ and Cs+.
Conclusions
This study permitted the characterisation of tetrahedrite-(Hg) as a specific mineral species belonging to the tetrahedrite group, in accordance with the nomenclature rules defined by Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020). It confirms the structural plasticity of the tetrahedrite structure, favouring the incorporation of a wide range of monovalent and divalent cations typical of hydrothermal ore deposits.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.36
Acknowledgements
CB and MP acknowledge financial support from the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32. This work was also supported (JS, DV) financially by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019-2023/1.II.b National Museum, 00023272). The comments of Yves Moëlo and two anonymous reviewers were greatly appreciated, helping us to improve the paper.