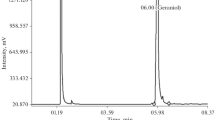
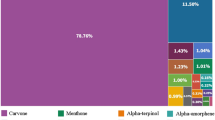
Issues related to reliable quality assessment of oil solutions of medicines with respect to the Bacterial Endotoxins indicator are considered. The maximum isolation of bacterial endotoxins from fat-soluble drugs for subsequent determination is assisted by vigorous stirring of samples in water previously heated to 60°C followed by separation of the two phases using centrifugation at 2300 rpm for 10 min.




Similar content being viewed by others
References
V. I. Chueshov, E. V. Gladykh, I. A. Lyapunova, et al., Technology of Industrially Manufactured Drugs [in Russian], (2010).
State Pharmacopoeia of the Russian Federation, XIVth Ed., GPM 1.2.4.0006.15 (2018).
R. A. Lavrenchuk, I. V. Sakaeva, and E. I. Sakanyan, Vedom. Nauchn. Tsentra Ekspert. Sredstv Med. Primen., No. 2, 40 – 42 (2012).
Yu. V. Merkulova, G. V. Dolgova, L. A. Chaika, et al., Farmakom, No. 1, 67 – 73 (2008).
A. A. Ievleva, V. A. Plisov, and E. Yu. Khramova, Handbook of Basic Drugs [in Russian], Moscow (2012).
Dandan Chen, J. Anal. Methods Chem., Art. ID 575246 (2014).
R. Pennamareddy, Thesis (2010), pp. 123 – 126.
M. B. Arambasic, Conference paper (2010).
M. B. Arambasic, Conference paper (2012).
Testing Oil Solutions with PYROGENT TM Gel Clot LAL Assay Technical Tips, Lonza Walkersville, Inc. (2013).
K. L. Williams, Endotoxins Pyrogens, LAL Testing and Depyrogenation, 3 rd Ed., (Drugs and the Pharmaceutical Sciences), CRC Press, (2007).
The necessity of LAL endotoxin testing, Blog (2014).
Acknowledgments
The work was performed in the framework of a State Task for SCEEMP, Ministry of Health of Russia, No. 056-00154-19-00 for applied scientific research (State Acct. No. NIR AAAA-A18-118021590049-0).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 5, pp. 58 – 61, May, 2020.
Rights and permissions
About this article
Cite this article
Shapovalova, O.V., Neugodova, N.P. & Sapozhnikova, G.A. Peculiarities of Determining Bacterial Endotoxins in Oil Solutions of Drugs. Pharm Chem J 54, 539–543 (2020). https://doi.org/10.1007/s11094-020-02234-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02234-7