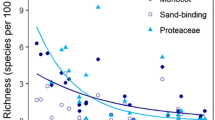
Abstract
Plants that produce specialised cluster roots, which mobilise large quantities of poorly available nutrients such as phosphorus (P), can provide a benefit to neighbouring plants that produce roots in the cluster rhizosphere, as demonstrated previously in pot studies. To be effective, such roots must be present within the short time of peak cluster activity. We tested if this requirement is met, and quantified potential P benefits, in a hyperdiverse Mediterranean woodland of southwest Australia where cluster-rooted species are prominent. Using minirhizotrons, we monitored root dynamics during the wet season in the natural habitat. We found non-cluster roots intermingling with all 57 of the observed cluster roots of the studied tree species, Banksia attenuata. Almost all (95%) of these cases were observed in a high-moisture treatment simulating the 45-year average, but not present when we intercepted some of the rainfall. We estimate that cluster-root activity can increase P availability to intermingling roots to a theoretical maximum of 80% of total P in the studied soil. Due to their high P-remobilisation efficiency (89%), which results from P rapidly being relocated from cluster roots within the plant, senesced Banksia cluster roots are a negligible P source for other roots. We conclude that, rather than serving as a P source, it is the cluster-root activity, particularly the exudation of carboxylates, that may improve the coexistence of interacting species that are capable of root intermingling, thus potentially promoting species diversity in nutrient-poor habitats, and that this mechanism will be less effective in a drying climate.





Similar content being viewed by others
Data accessibility
Data will be made available as part of a separate digital file in the Electronic Supplementary Material section (Data S1).
References
Abrahão A et al (2019) Soil types select for plants with matching nutrient-acquisition and -use traits in hyperdiverse and severely nutrient-impoverished campos rupestres and cerrado in Central Brazil. J Ecol 107:1302–1316
Beeck D (2017) Cluster-root exudation of carboxylate and phenolic compounds by two species of Banksia. Honours thesis, The University of Western Australia, Crawley, WA, Australia
Cameron AC, Trivedi PK (2005) Microeconometrics: methods and applications. Cambridge University Press, Cambridge
Chen W, Koide RT, Eissenstat DM (2018) Root morphology and mycorrhizal type strongly influence root production in nutrient hot spots of mixed forests. J Ecol 106:148–156. https://doi.org/10.1111/1365-2745.12800
Crosti R, Dixon KW, Ladd PC, Yates CJ (2007) Changes in the structure and species dominance in vegetation over 60 years in an urban bushland remnant. Pacif Conserv Biol 13:158–170
Delgado M, Zúñiga-Feest A, Alvear M, Borie F (2013) The effect of phosphorus on cluster-root formation and functioning of Embothrium coccineum (R. et J. Forst.). Plant Soil 373:765–773
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007a) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30:1557–1565
Denton MD, Veneklaas EJ, Lambers H (2007b) Does phenotypic plasticity in carboxylate exudation differ among rare and widespread Banksia species (Proteaceae)? New Phytol 173:592–599
Diffenbaugh NS, Field CB (2013) Changes in ecologically critical terrestrial climate conditions. Science 341:486–492
Faget M et al (2013) Root-root interactions: extending our perspective to be more inclusive of the range of theories in ecology and agriculture using in-vivo analyses. Ann Bot 112:253–266. https://doi.org/10.1093/aob/mcs296
Falik O, Mordoch Y, Quansah L, Fait A, Novoplansky A (2011) Rumor has it: relay communication of stress cues in plants. PLoS ONE 6:1–6
Fisher JL, Loneragan WA, Dixon K, Delaney J, Veneklaas EJ (2009) Altered vegetation structure and composition linked to fire frequency and plant invasion in a biodiverse woodland. Biol Conserv 142:2270–2281
Gardner WK, Boundy KA (1983) The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species. Plant Soil 70:391–402
George K, Norby R, Hamilton J, DeLucia E (2003) Fine-root respiration in a loblolly pine and sweetgum forest growing in elevated CO2. New Phytol 160:511–522
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gorman ML, Mills MG, Raath JP, Speakman JR (1998) High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391:479–481
Helmisaari H-S, Saarsalmi A, Kukkola M (2009) Effects of wood ash and nitrogen fertilization on fine root biomass and soil and foliage nutrients in a Norway spruce stand in Finland. Plant Soil 314:121–132. https://doi.org/10.1007/s11104-008-9711-4
Hodge A (2009) Root decisions. Plant Cell Environ 32:628–640. https://doi.org/10.1111/j.1365-3040.2008.01891.x
Hodge A (2012) Plant root interactions. In: Witzany G, Baluska F (eds) Biocommunication of plants, signaling and communication in plants, vol 14. Springer, Berlin, pp 157–169
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650
Initiative IOC (2012) Western Australia’s weather and climate: a synthesis of Indian Ocean Climate Initiative stage 3 research. Commonwealth of Australia, Melbourne
Jeschke WD, Pate JS (1995) Mineral nutrition and transport in xylem and phloem of Banksia prionotes (Proteaceae), a tree with dimorphic root morphology. J Exp Bot 46:895–905
Johnson MG, Tingey DT, Phillips DL, Storm MJ (2001) Advancing fine root research with minirhizotrons. Environ Exp Bot 45:263–289. https://doi.org/10.1016/s0098-8472(01)00077-6
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll KH, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2‐million‐year dune chronosequence in the south‐western Australia biodiversity hotspot. J Ecol 100:631–642
Lambers H (ed) (2014) Plant life on the sin Southwest Australia, a global biodiversity hotspot. University of Western Australia Publishing, Crawley, Australia
Lambers H, Oliveira RS (2019) Plant physiological ecology, 3rd edn. Springer, Cham
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible W-R, Stitt M, Teste FP, Turner BL (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108. https://doi.org/10.1111/j.1469-8137.2012.04285.x
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100:263–288
Lambers H, Shane M, Laliberté E, Swarts N, Teste F, Zemunik G (2014) Plant Mineral Nutrition. In: Lambers H (ed) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. UWA Publishing, Crawley, pp 101–127
Lambers H, Clode PL, Hawkins H-J, Laliberté E, Oliveira RS, Reddell P, Shane MW, Stitt M, Weston P (2015) Metabolic adaptations of the non-mycotrophic proteaceae to soils with low phosphorus. In: Plaxton W, Lambers H (eds) Annual plant reviews. Phosphorus metabolism in plants, vol 48. Wiley, pp 289–336
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, Zemunik G (2018) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 424:11–33. https://doi.org/10.1007/s11104-017-3427-2
Lamont BB (1976) The effects of seasonality and waterlogging on the root systems of a number of Hakea species. Aust J Bot 24:691–702
Lamont B (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. Bot Rev 48:597–689
Lamont BB (2003) Structure, ecology and physiology of root clusters—a review. Plant Soil 248:1–19
Li L, Li S-M, Sun J-H, Zhou L-L, Bao X-G, Zhang H-G, Zhang F-S (2007) Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc Nat Acad Sci 104:11192–11196
Li L, Tilman D, Lambers H, Zhang FS (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203:63–69
Li B, Li Y-Y, Wu H-M, Zhang F-F, Li C-J, Li X-X, Lambers H, Li L (2016) Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc Nat Acad Sci 113:6496-6501. https://doi.org/10.1073/pnas.1523580113
McArthur WM, Bettenay E (1974) The development and distribution of the soils of the Swan Coastal Plain. CSIRO, Australia
McIntire EJB, Fajardo A (2014) Facilitation as a ubiquitous driver of biodiversity. New Phytol 201:403–416. https://doi.org/10.1111/nph.12478
McPharlin I, Jeffery R, Weissberg R (1994) Determination of the residual value of phosphate and soil test phosphorus calibration for carrots on a Karrakatta sand. Commun Soil Sci Plant Anal 25:489–500
Metzner R, Eggert A, van Dusschoten D, Pflugfelder D, Gerth S, Schurr U, Uhlmann N, Jahnke S (2015) Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods. https://doi.org/10.1186/s13007-015-0060-z
Mommer L, Kirkegaard J, van Ruijven J (2016) Root–root interactions: towards a rhizosphere framework. Trends Plant Sci 21:209–217
Muler AL, Oliveira RS, Lambers H, Veneklaas EJ (2014) Does cluster-root activity benefit nutrient uptake and growth of co-existing species? Oecologia 174:23–31
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Oburger E, Schmidt H (2016) New methods to unravel rhizosphere processes. Trends Plant Sci 21:243–255. https://doi.org/10.1016/j.tplants.2015.12.005
Ostonen I, Lõhmus K, Pajuste K (2005) Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: comparison of soil core and ingrowth core methods. For Ecol Manage 212:264–277
Ozanne P, Shaw T (1968) Advantages of the recently developed phosphate sorption test over the older extractant methods for soil phosphate. In: Holmes JW (ed) Transactions of the 9th International Congress of Soil Science, vol 2. Angus and Robertson. Sydney, Australia, pp 273–280
Pate JS, Beard JS (1982) Kwongan, plant life of the sandplain. University of Western Australia Press, Perth
Pate JS, Dell B (1984) Economy of mineral nutrients in sandplain species. In: Pate JS, Beard JS (eds) Kwongan, plant life of the sandplain. University of Western Australia Press, Nedlands, pp 227–252
Pate JS, Watt M (2002) Roots of Banksia spp. (Proteaceae) with special reference to functioning of their specialized proteiod root clusters. In: Eshel A, Beeckman T (eds) Plant roots: the hidden half. Marcel Dekker Inc., New York, pp 989–1006
Pathan S, Aylmore L, Colmer T (2003) Soil properties and turf growth on a sandy soil amended with fly ash. Plant Soil 256:103–114
Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler JP (2014) Biogenic volatile emissions from the soil. Plant Cell Environ 37:1866–1891. https://doi.org/10.1111/pce.12340
Peterson RL, Peterson CA, Melville LH (2008) Teaching plant anatomy: through creative laboratory exercises. NRC Research Press, Ottawa
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Saunders WMH, Williams EG (1955) Observatioms on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739. https://doi.org/10.1111/j.1365-2745.2006.01124.x
Schmittgen S et al (2015) Magnetic resonance imaging of sugar beet taproots in soil reveals growth reduction and morphological changes during foliar Cercospora beticola infestation. J Exp Bot 66:5543–5553. https://doi.org/10.1093/jxb/erv109
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135:549–560
Shi J, Strack D, Albornoz F, Han Z, Lambers H (2020) Differences in investment and functioning of cluster roots account for different distributions between Banksia attenuata and B. sessilis, with contrasting life history. Plant Soil 447:85–98
Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (2000) Root methods: a handbook. Springer, Berlin
Teodoro GS et al (2019) Specialized roots of Velloziaceae weather quartzite rock while mobilizing phosphorus using carboxylates. Funct Ecol 33:762–773
Teste FP, Veneklaas EJ, Dixon KW, Lambers H (2014) Complementary plant nutrient-acquisition strategies promote growth of neighbour species. Funct Ecol 28:819–828
Teste FP, Marchesini VA, Veneklaas EJ, Dixon KW, Lambers H (2018) Root dynamics and survival in a nutrient-poor and species-rich woodland under a drying climate. Plant Soil 424:91–102. https://doi.org/10.1007/s11104-017-3323-9
Therneau T (2015) A package for survival analysis in S. version 2.38, R package version 2.38 edn
Thompson D (1986) The economics of kleptoparasitism: optimal foraging, host and prey selection by gulls. Anim Behav 34:1189
Turner BL, Romero TE (2009) Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Sci Soc Am J 73:1972–1979
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
van Dusschoten D, Metzner R, Kochs J, Postma JA, Pflugfelder D, Buhler J, Schurr U, Jahnke S (2016) Quantitative 3D analysis of plant roots growing in soil using magnetic resonance imaging. Plant Physiol 170:1176–1188. https://doi.org/10.1104/pp.15.01388
van Vuuren MMI, Robinson D, Griffiths BS (1996) Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in soil. Plant Soil 178:185–192
Watson AP, Matthiessen JN, Springett BP (1982) Arthropod associates and macronutrient status of the red‐ink sundew (Drosera erythrorhiza Lindl.). Aust J Ecol 7(1):13–22
Watt M, Evans JR (1999) Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiol 120:705–716
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Williams JDH, Syers JK, Walker TW, Rex RW (1970) A comparison of methods for the determination of soil organic phosphorus. Soil Sci 110:13–18
Wright AJ, Wardle DA, Callaway R, Gaxiola A (2017) The overlooked role of facilitation in biodiversity experiments. Trends Ecol Evol 32:383–390
Wyrwoll K-H, Turner BL, Findlater P (2014) 1a. On the origins, geomorphology and soils of the sandplains of south-western Australia. In: Lambers H (ed) Plant Life on the Sandplains in Southwest Australia, a Global Biodiversity Hotspot. UWA Publishing, Crawley, pp 3–22
Yu RP, Zhang WP, Yu YC, Yu SB, Lambers H, Li L (2020a) Linking shifts in species composition induced by grazing with root traits for phosphorus acquisition in a typical steppe in Inner Mongolia. Sci Total Environ 712:136495. https://doi.org/10.1016/j.scitotenv.2020.136495
Yu R-P, Li X-X, Xiao Z-H, Lambers H, Li L (2020b) Phosphorus facilitation and covariation of root traits in steppe species. New Phytol 226:1285–1298
Zeng G, Birchfield ST, Wells CE (2010) Rapid automated detection of roots in minirhizotron images. Mach Vis Appl 21:309–317
Zomora R (1995) The trapping success of a carnivorous plant, Pinguicula vallisneriifolia: the cumulative effects of availability, attraction, retention and robbery of prey. Oikos 73:309–322
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
Michael Blair and Raymond Scott provided help at the start of the experimental setup and tube installations and for facilitating access to the field station. We are grateful to Jairo Palta, then at CSIRO in Floreat, for lending us the Bartz minirhizotron camera. Thomas Mazet played a key role with imaging during rainy days. We are especially grateful to Judith Holmes and Victoria A. Marchesini for their valuable help during the root annotation with RootFly. We thank Michael Smirk and Katrina Walton for their help quantifying nutrient concentrations of cluster roots. Finally, we thank Simone Pedrini for the illustrations of the cluster roots in Fig. 5. Funding was provided by The University of Western Australia with a Research and Development Award granted to FPT and the Australian Research Council with a Discovery Project (ARC DP0985685) to HL, EJV and KWD. KWD and EJV are also recipients of an Australian Research Council (ARC) Industrial Transformation Training grant for the Centre for Mine Site Restoration (Project Number ICI150100041).
Author information
Authors and Affiliations
Contributions
FPT, EJV, KWD, and HL designed the study. FPT analysed the data and wrote the first draft of the manuscript and all authors collected data and contributed substantially to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Mercedes Bustamante.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teste, F.P., Dixon, K.W., Lambers, H. et al. The potential for phosphorus benefits through root placement in the rhizosphere of phosphorus-mobilising neighbours. Oecologia 193, 843–855 (2020). https://doi.org/10.1007/s00442-020-04733-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04733-6