Abstract
Shell formation and repair occurs under the control of mantle epithelial cells in bivalve molluscs. However, limited information is available on the precise acid–base regulatory machinery present within these cells, which are fundamental to calcification. Here, we isolate mantle epithelial cells from the Pacific oyster, Crassostrea gigas and utilise live cell imaging in combination with the fluorescent dye, BCECF-AM to study intracellular pH (pHi) regulation. To elucidate the involvement of various ion transport mechanisms, modified seawater solutions (low sodium, low bicarbonate) and specific inhibitors for acid–base proteins were used. Diminished pH recovery in the absence of Na+ and under inhibition of sodium/hydrogen exchangers (NHEs) implicate the involvement of a sodium dependent cellular proton extrusion mechanism. In addition, pH recovery was reduced under inhibition of carbonic anhydrases. These data provide the foundation for a better understanding of acid–base regulation underlying the physiology of calcification in bivalves.
Similar content being viewed by others
Introduction
Shells of the Pacific oyster, Crassostrea gigas, a mollusc species with enormous economic and ecological value (Zhang et al. 2012) are characterized by the presence of two calcareous valves. In adult C. gigas molluscs, shell formation occurs under the control of the mantle tissue, where mantle epithelial cells take part in the transport of calcification substrates (Gong et al. 2008) (Ca2+ and HCO3−). Recently, Sillanpaa et al. (2018) reported that up to 60% of the Ca2+ transport in C. gigas mantle epithelia occurs via the transcellular pathway. However, it is unknown at present by which pathways bivalve molluscs control the precipitation of the calcareous shell. Since proton production is a by-product of mineral formation from the bicarbonate (HCO3−) substrate (Zeebe and Wolf-Gladrow 2001), specific mechanisms have to be present in calcifying molluscs to minimize fluctuations in intracellular pH (pHi). For calcification to take place, mantle epithelial cells would be responsible for generating and maintaining a highly regulated microenvironment. This makes intracellular pH homeostasis intricately linked to biomineralization as HCO3− and Ca2+ import will require the export or buffering of protons to maintain pHi.
Regulation of pHi is a key aspect of cell physiology and cells make use of evolutionarily conserved membrane-bound transport proteins or intracellular buffering to accomplish stable pHi (Laurent et al. 2014). Membrane-bound transport proteins can achieve proton extrusion (sodium/hydrogen exchangers (NHEs), V-type proton ATPases), bicarbonate uptake (sodium driven bicarbonate transporters) and most importantly generate the electrochemical gradient driving pHi regulation (sodium–potassium ATPases). In addition to pHi regulation, these membrane-transporters are also crucial for calcification by providing the substrates necessary through cellular ion transport. This occurs via primary (calcium-ATPases) and secondary (sodium/calcium exchangers and sodium driven chloride/bicarbonate exchangers) pathways. Additionally, membrane transporters also aid in the removal of proton by-products from the calcification substrate HCO3− (Ramesh et al. 2019). Molecular evidence for the presence of such transporters in molluscs comes from cloning of calcium transporting proteins within the mantle tissue in the pearl oyster, Pinctada fucata and the observed expression of all of the above-mentioned pHi regulatory proteins in several mollusc species (Wang et al. 2008; Zhang et al. 2012; De Wit et al. 2018). Maintenance of stable pHi has been demonstrated for a range of muscle types including cardiac myocytes and adductor and byssus retractor muscles (Ellington 1983, 1993; Zange et al. 1990). In C. gigas, pHi regulation in such non-calcifying tissues has been shown to be dissimilar to the mantle, as cardiac tissues exhibit intrinsically higher intracellular non-bicarbonate, non-phosphate buffer capacities (Michaelidis et al. 2005). Regulation of pHi can also be achieved via intracellular modulation of bicarbonate concentrations via carbonic anhydrases and a range of other proton equivalent exchange processes. Carbonic anhydrases can produce HCO3− from intracellular CO2 and their role in biomineralisation has been studied in a range of molluscs (Medakovic 2000; Marie et al. 2008). However, there is limited information regarding the role of carbonic anhydrases in pHi regulation, particularly in calcifying tissue.
Characterization of the ability of bivalve mantle cell acid–base regulatory mechanisms during induced stress, is rudimentary, although knowledge of the pHi control mechanisms in bivalve models such C. gigas is particularly important in the context of anthropogenic seawater acidification. Anthropogenic seawater acidification is accompanied with elevated dissolved carbon dioxide which consequently alters extracellular acid–base homeostasis (Zlatkin and Heuer 2019; Melzner et al. 2020). Similar to many molluscs, oysters are considered to be weak extracellular acid–base regulators and can only partially compensate for induced acidosis (Dwyer and Burnett 1996). Therefore, the relationship between seawater acidification, and pHi regulation is intricate and our ability to accurately predict the response of calcifying bivalves to seawater acidification is severely hindered by our limited understanding of the cellular mechanisms driving pH homeostasis in these organisms.
Here, we use primary mantle cell cultures to investigate the previously uncharacterized pHi regulatory capacity of mantle epithelial cells in C. gigas using live cell imaging and the pH-sensitive fluorescent probe, 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM). Furthermore, we utilize pharmaceutical blockers and modifications in ion composition to investigate the role of key ion transport pathways to provide crucial information on the mechanisms of pHi regulation in molluscan calcifying tissue.
Materials and methods
Oyster collection and maintenance
Adult aquaculture raised C. gigas were purchased from Dittmeyer’s Austern Compagnie GmbH (‘Sylter Royal’, List, Germany) and delivered over night to GEOMAR Helmholtz Centre for Ocean Research Kiel. Animals were utilized for experiments within 30 days following arrival. Animals were maintained at a temperature of 11 °C and pHNBS of 8.0 ± 0.1 in an aerated, re-circulating seawater system without feeding in seawater prepared using Instant Ocean to a salinity of 31 ± 2 psu. Water in the culture system was exchanged on a weekly basis. Ammonium concentrations were tested using a JBL NH4+ aquaria kit and maintained below a concentration of 0.05 mg/L.
Mantle cell culture
Primary mantle cells were cultured according to Gong et al. (2008) with slight modifications. Briefly, oysters were dissected, and pallial mantle tissue as described in Gong et al. (2008) was excised for cell culture. The mantle tissue was sanitized for 20 min in an artificial seawater solution (ASW, Supplementary Table 1) containing 0.5 mg/ml streptomycin, 500 IU/ml penicillin,100 IU/ml gentamicin, and 2 μg/ml nystatin. After rinsing 3 times in a calcium and magnesium free ASW (Supplementary Table 1), the pallial mantle tissue was minced into small fragments (approx. 2 mm diameter) and these fragments were planted onto the center of lysine coated glass coverslips (Eydam, Germany), placed into sterile petridishes. Cell cultures were maintained in a culture medium that is detailed in Supplementary Table 2. Cells of interest were allowed to migrate out of explants for 24 h, explants were removed and sedentary culture cells remaining on the coverslips were used for microfluorimetry. All reagents were purchased from Sigma-Aldrich unless otherwise specified.
Preparation of solutions
Artificial seawater (ASW) solutions were prepared according to Zeebe and Wolf-Gladrow (2001) (Supplementary Table 1). Osmolality (1104 ± 5 mOsm kg−1) and salinity (31 ± 2 psu) were selected to match the seawater values in the culture system (1113 ± 8 mOsm kg−1). Inhibitors were dissolved in DMSO and added at final concentrations of 20 µM (ethylisopropyl amiloride, EIPA) and 1 mM (acetazolamide, ACZM) to ASW. DMSO concentrations did not exceed 0.1%.
BCECF dye loading
To measure mantle epithelial cell pHi, cover slips containing cell cultures were affixed to glass perfusion chambers (Supplementary Fig. 1) using a hydrophobic, silicone gel and bathed in ASW with a final BCECF-AM concentration of 10 μM at 19 °C for 30 min in the dark. Following dye loading, cells that were firmly attached to the coverslip were used for measurements. The flow rate of the perfusion system was 1–2 mL min−1 and experiments were performed at 19 °C.
Microfluorimetry
Microfluorimetric measurements were performed on an inverted microscope (Zeiss Axio Observer. D1) equipped with a 40 × objective (Zeiss) and a CoolSNAP HQ2 CCD camera (Photometrics, USA). The dye was excited alternatively at two wavelengths, 486 nm and 439 nm (± 10 nm bandwidth) for 24 and 60 ms, respectively. Emission was recorded at 525 nm and fluorescence was monitored with the imaging system Visitron. The ratio of the emission intensities at the two excitation wavelengths over mantle cell was calculated, following background subtraction of camera offset using the software Metafluor 7.6.1. From each coverslip, the recordings of one to six mantle epithelial cells were collected and averaged. For each treatment, between 4 and 7 individual oysters were used as biological replicates. The ionophore nigericin was used to calibrate pHi of mantle cells as previously described by Stumpp et al. (2012). Mantle cells were exposed to 10 μM nigericin in the presence of 160 mM potassium [K+] at pH 6.5, 7.0, 7.5 and 8.0. This K+ concentration was chosen to be in the range of intracellular [K+] reported for marine molluscs (Potts 1958; Ellington 1993). The calibration curve allowed calculation of the relationship between recorded emission ratio of BCECF and the corresponding pHi. For pHi recovery experiments, mantle cells were exposed to ASW for 10 min followed by a 20 mM NH3/NH4+ pulse. Alkalosis compensation rates were calculated as the slope during this ammonia prepulse phase and are indicative of the rate of active pHi acidification during the NH3/NH4+ prepulse (Table 1). Acidosis was consecutively induced by the washout of NH3/NH4+ using the following solutions: ASW as control condition and 5 mM Na+, low HCO3− or ASW plus inhibitors (see above) to assess the involvement of different ion transport systems. Consecutive experiments (control followed by treatment experiments) were not performed due to the required length of such experiments and therefore, the current experimental design is associated with natural differences in cell-to-cell variability. Recovery rates were estimated from the compensatory slope after induced acidosis for the linear phase of recovery marked by the red lines in Fig. 2.
Buffer capacity
Buffer capacity (β) was estimated using the NH3/NH4 pulse as described by Boron (1977) and is expressed as Slykes (mM/pH unit). Concentrations of NH4+ in ASW were assumed to be negligible and nominally set to zero (Boron 1977). Following NH3/NH4+ pulse, β was calculated with the following formula:
Data analysis
All data were analysed using R (Version 3.3.2, R Development Core Team, R: http://www.R.org/. 2011). Data were tested for normality and homogeneity using Shapiro-Wilks test and Bartlett test, respectively. If assumption for normality was not met, data were transformed by applying Box-Cox transformations. To determine the ability of mantle epithelial cells to recover from an NH3/NH4+ induced acidosis, the alkalosis compensation rates, pHi recovery rates and final pHi (after 60 min) were tested for fixed effects of washout solution and the random effects of animals as replicate. A mixed effects model using the lmer function in the lmerTest package was applied and significant effects were determined using the ANOVA function. Post hoc analyses were performed via Tukey HSD tests. Data on Δ[H+] were analysed using a Kruskal–Wallis test followed by a Dunn’s posthoc test.
Results
Mantle epithelial cell culture
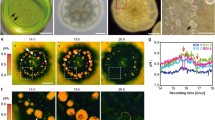
Following 24 h of cultivation, three typical cell populations were commonly observed around mantle explants, namely mantle epithelial cells, granular hemocytes and hyalinocytes (agranular hemocytes) (Fig. 1a–d). In addition, certain cultures contained the presence of spindle-like muscle cells (not shown). Cells were identified based on size, morphology and characteristic movement of the two hemocyte cell types as described previously (Awaji 1991; Gong et al. 2008). Although hemocytes have been linked to calcification in oysters (Mount et al. 2004; Ivanina et al. 2017), microfluorimetric measurements were not performed for these cell types due to a vesicular concentration of BCECF in addition to the cytosolic signal. Regions of the cell cultures containing higher abundance of the roundish, stationary epithelial cells were selected for microfluorimetric measurements.
A Phase contrast image of mantle tissue cell culture in Crassostrea gigas depicting an epithelial cell (e), granular hemocytes (g) and hyalinocytes (h). Higher magnification images of all the three cell types labelled in (A): (B) epithelial cell (C) granular hemocytes (D) hyalinocyte. Scale bars: 20 μm in (A), 40 μm in (B), 30 μm in (C) and 50 μm in (D). E Fluorometric pHi measurement in a mantle epithelial cell. F Calibration curve of BCECF-AM in mantle epithelial cells of Crassostrea gigas allowing the conversion of ratios to pHi values. Dots represent individual cells
pH regulatory proteins in oyster mantle epithelia
Figure 1e shows a typical fluorometric measurement of one single mantle epithelial cell under resting conditions and after challenging the pHi by an ammonium pulse experiment. Cells where perfused with ASW (resting, control situation). Superfusion with 20 mM of the mild base NH3/NH4+ leads to alkalization (alkalosis) with slight counter-reaction of the cells (blue line), representing the alkalosis compensation rate, which was not significantly different between experiments (p > 0.05). This prepulse was followed by an acidosis phase upon wash-out of NH3/NH4+ leaving protons behind which have been produced as a result of alkalosis compensation. The following recovery phase (recovery) can be used to estimate proton extrusion and buffer capacity in cells (red lines in Fig. 2). Nigericin calibration was used to translate ratio into pHi (Fig. 1f). N = 4–7 experiments were summarized for each of the measuring conditions in the following figures. An experiment was defined as cells isolated from N = 1 oyster. Figure 2a shows the control experiment where the wash-out was done by ASW (control). Cells maintained a resting pHi of 6.84 ± 0.04. This pHi was found to be stable for at least 180 min (Supplementary Fig. 2a, shown for one cell) and was used to establish cell culture status for further experiments. When cells were perfused with 20 mM NH3/NH4+, pHi increased to a value of 7.45 ± 0.04. Removal of 20 mM NH3/NH4+ and perfusion with ASW induced an intracellular acidosis (pHi 6.54 + 0.04) followed by gradual recovery in pHi to 6.96 ± 0.07. A recovery rate (red line) of approx. 0.01 pH units/min was estimated (Fig. 2) for control conditions. Baseline pHi for the different cell preparations varied under resting conditions and this variation may be biological or depend on seasonality. For this reason, we investigated rates of pHi recovery following acidosis and Δ[H+] between resting and recovery phases. Figure 2b, c summarize the results for experiments where the washout of NH3/NH4+ was performed under low Na+ (B) and low bicarbonate (C) conditions where pHi recovery in oyster mantle epithelial cells are significantly inhibited by modification of ASW in comparison to control experiments (ANOVA, F = 18.17, p < 0.05). Specifically, ASW treatments containing reduced Na+ or pharmacological inhibitors of the NHE (EIPA) resulted in significantly lower rates of pHi recovery (Fig. 3, Tukey HSD, p < 0.05 respectively). To consider potential effects of DMSO, resting pH (prior to administration of inhibitors) between control and the two inhibitor experiments were examined and no significant differences were observed (p > 0.05, one-way ANOVA). Cell vitality following perfusion with these modified ASW solutions was confirmed by observing recovery to resting pHi upon addition of control ASW containing comparable concentrations of DMSO (Supplementary Fig. 2b). In addition, rates of pHi recovery were decreased when mantle epithelial cells were perfused with the carbonic anhydrase inhibitor, ACZM (Tukey HSD, p < 0.05). No significant effect on pHi recovery rates was observed when mantle epithelial cells were perfused in ASW containing low HCO3− (Tukey HSD, p > 0.05). However, final pHi values following recovery period from the ammonium prepulse were significantly different in experiments where mantle epithelial cells were perfused with modified ASW solutions (ANOVA, F = 6.46, p < 0.05) where, experiments in the presence of low Na+ (Tukey HSD, p < 0.01) and EIPA (Tukey HSD, p < 0.01) revealed significantly lower pHi at 60 min. Similarly, Δ[H+] were found to be significantly different (Kruskal–Wallis, Χ2 = 24.01, p < 0.05), where significant differences were found for the low HCO3−, low Na+ and EIPA washouts (Dunn’s Test, p < 0.05). Recovery rates ([H+]/minute) were − 3.67E − 08 ± 7.82E − 09, − 4.1E − 08 ± 4.33E − 09, − 3.98E − 09 ± 1.75E − 09, − 1.22E − 08 ± 2.53E − 09 and − 5.49E − 09 ± 9.65E − 10 for ASW, low bicarbonate, low Na+, EIPA and acetazolamide washouts respectively (Supplementary Fig. 4).
Acid-base regulatory capacities of mantle epithelial cells in Crassostrea gigas under the presence of various ASW solutions prepared according to Supplementary Table 1 and pharmacological inhibitors of specific cellular ion transport proteins. pHi recordings in the presence of a ASW (control recovery) b low HCO3− c low Na+ d 1 mM acetazolamide and e 20 µM EIPA. Values are presented as mean ± SEM for various replicates as described in Table 1. Blue and red lines indicate slope of alkalosis and acidosis compensation respectively (color figure online)
a Recovery rates of pHi and b remaining difference in protons (Δ[H+]) during washout phase in Crassostrea gigas epithelial cells when exposed to various ASW solutions or pharmacological inhibitors of specific cellular ion transport proteins. Data are presented as mean ± SEM for various replicates as described in Table 1 and asterisks represent significant differences from control as tested by an ANOVA
Determination of buffer capacity (β)
We estimated β by perfusing mantle epithelial cells with 20 mM NH3/NH4+ followed by control ASW and observed a mean β value of 22.53 ± 1.26 mM/pH unit (n = 11 cells). Between experimental groups, within this short experimental period, calculated β was not different (Table 1).
Discussion
Mollusc mantle epithelia have been long been studied to elucidate the biological control of calcification (Neff 1972; Joubert et al. 2010; Herlitze et al. 2018). It is crucial to understand the fundamental cellular acid–base regulatory pathways utilized by these cells to secrete calcified shells. The stable average pHi of approx. 6.8 recorded by in vivo fluorescence imaging is comparable to those reported in other molluscan cells, including C. gigas hemocytes (Beckmann 2011; Michaelidis et al. 2005). Ammonium prepulse experiments demonstrate that oyster mantle epithelial cells have the capacity to recover from cellular acid load by a sodium dependent, EIPA-sensitive pathway. Additionally, we describe the buffer capacity (β) of calcifying mantle epithelial cells which may shed light on the ability of these cells to regulate pHi when exposed to environmental hypercapnia and ongoing ocean acidification.
Using the ammonium prepulse technique, we observe a consistent cellular acidosis below control pHi in C. gigas mantle epithelial cells, down to pHi 6.54. This acidosis was followed by a recovery phase back to stable pHi values around 6.96 (Table 1). Similar observations have been observed in other mollusc cell types including cardiac myocytes, oyster hemocytes and limpet oocytes (Ellington 1993; Vilain et al. 1993; Beckmann 2011). Our results demonstrate that the rates of pHi recovery are significantly lower when cells are perfused with modified ASW solutions containing low Na+ or a pharmacological inhibitor, EIPA (Fig. 2, 3). The involvement of NHE proteins in pHi regulation in mussel hemocytes and isolated mantle/gonad cells has been observed utilising pharmacological techniques (Kaloyianni et al. 2005; Banakou and Dailianis 2010). This group of ion-regulatory proteins has also previously been observed to exhibit upregulated mRNA expression in the mantle of the pearl oyster, Pinctada fucata during seawater acidification (Li et al. 2016). Within the genus Crassostrea, there are multiple genes encoding NHE transport proteins with similarity to cell membrane and intracellular (mitochondrial) isoforms in Homo sapiens and Mus musculus. However, an elementary molecular description for this group of antiporter proteins has been conducted in only one mollusc species, the giant clam, Tridacna squamosa, where NHE-3 isoforms have a predicted role in calcification (Hiong et al. 2017). This group of proteins has also been demonstrated to be crucial in the tolerance to seawater acidification in another mollusk, the squid, Sepioteuthis lessoniana (Hu et al. 2013, 2014). Taken together with the results from the present study, these observations indicate that NHE’s which are sensitive to EIPA are crucial to pHi regulation in C. gigas mantle epithelial cells. Rates of pHi recovery in C. gigas mantle epithelial cells are ca. 10 times slower than recovery rates observed in hemocytes within the same species (Beckmann 2011) but are comparable to recovery rates observed in cnidarian cells (Laurent et al. 2014), where NHE pathways have also been established to be important for pHi regulation. Additionally, the compensation of induced alkalosis was very weak in oyster mantle epithelial cells in comparison to mammalian cells types (Bourgeois et al. 2018). This may be because these cells rarely experience such an extracellular alkalosis in the environment (seawater) or hemolymph and similar weak compensations to an ammonia induced alkalosis have also been observed in sea urchin larvae (Stumpp et al. 2012; Hu et al. 2018).
In addition to proton extrusion pathways mediated by NHEs and other proteins, cellular mechanisms of bicarbonate (HCO3−) transport can also play an important role in pHi homeostasis. Our experiments indicate that modified ASW containing low HCO3− has no significant effect on the rates of pHi recovery and final pHi values following induced cellular acidosis. However, it has to be mentioned here that little is known regarding the bicarbonate affinity of bicarbonate transporters in molluscs. Typically, bicarbonate affinities of acid/base proteins in marine invertebrates are matched to their environment and/or extracellular fluids (Tresguerres 2014). In the present study, the low HCO3− solution was measured to contain 158 μmol kg−1 seawater HCO3− and therefore, we cannot exclude the possibility of residual transport enabled by the minor fractions of HCO3−. In contrast to the results obtained on the role of extracellular HCO3− in C. gigas pHi regulation in mantle cells, significantly lower pHi recovery rates in the presence of the carbonic anhydrase inhibitor, acetazolamide were observed. The enzyme carbonic anhydrase facilitates the reversible hydration of CO2 to HCO3− and has long been suggested to be an important enzyme in mollusc shell forming tissue such as mantle epithelia (Medakovic 2000; Miyamoto et al. 2005; Yu et al. 2006; Aguilera et al. 2017). Seventeen genes encoding both extracellular and cytosolic isoforms of carbonic anhydrase proteins have been identified in the C. gigas genome (Zhang et al. 2012). Recent molecular cloning and characterisation of carbonic anhydrase II in C. gigas has revealed that this protein has highly conserved catalytic domains, is expressed in all tissues and its inhibition affects pHi homeostasis (Wang et al. 2017). Further, in oysters, this carbonic anhydrase isoform is localised to the outer epithelia of mantle tissue and is observed to exhibit significant mRNA upregulation in response to CO2 exposure (Li et al. 2016; Wang et al. 2017).
Our results demonstrate that the activity of specific ion regulatory proteins such as NHEs and carbonic anhydrase are crucial for acid–base regulation. Interestingly, these proteins have also been associated with biomineralization (Medakovic et al. 2000; Zhang et al. 2012). Specifically, a suite of novel molecular studies lend support to the role of these carbonic anhydrases in acquisition of inorganic carbon during calcification (Wang et al. 2017; Koh et al. 2018; Chew et al. 2019). Additionally, it has been suggested that NHE proteins promote calcification by aiding in the removal of proton byproducts (Hiong et al. 2017; Cao-Pham et al. 2019). In bivalves, NHEs exhibit peaks in gene expression at the onset of larval calcification (Ramesh et al. 2019) and are also implicated in adult shell formation based on shell Na:Ca ratios (Zhao et al. 2017a, b). The concurrent activity of NHE and sodium/calcium exchange (NCX) proteins (Na+-dependent elevation of calcium) in mollusc calcification has not been studied. However, apical NCX proteins are suggested to be involved in calcium transfer across the oyster mantle epithelia (Sillanpaa et al. 2018) and whether Na+ exchange for calcium occurs following NHE mediated Na+ entry requires validation.
Although the role of ion transport is pivotal in pHi homeostasis, cells may also minimize the effects of extracellular pH change through their cellular buffering capacity (β). The β of a specific cell type is related to the osmotic pressure, [HCO3−], glycolysis, sensitivity to pH and cation disturbances, the degree to which cells have the role of buffering the extracellular fluid and concentration of compounds containing histidine residues (Burton 1978; Abe 2000). In the present study, we used the NH3/NH4+ prepulse technique to determine β and observed a mean value of 22.53 Slykes, which is in the range reported for other molluscan cell types such as snail neurons and whelk radula muscle (25 Slykes, Thomas 1974 and 30 Slykes, Wiseman and Ellington 1989). However, oyster hemocytes within the same species have been observed to exhibit distinctly lower β (8 Slykes, Beckmann 2011). The relatively high β in C. gigas mantle epithelial cells may be consistent with the necessity of these cells to protect themselves from acid load during calcification, where protons are generated as byproducts.
In the context of global environmental change, there is little information on plasticity of pHi regulation in these calcifying cells. One study that indirectly estimates pHi in hemocytes suggests that C. gigas elevates pHi upon CO2 exposure (Wang et al. 2016). Further, in response to seawater acidification, oysters have demonstrated an increased metabolic demand for NHEs which has been associated with increased proton extrusion (Stapp et al. 2018). Simultaneously, seawater acidification has been linked to an increased elimination of metabolic CO2 in oysters, a potential resilience mechanism (Stapp et al. 2018). However, without direct measurements, it is difficult to estimate the degree to which these organisms can respond to seawater pH reductions, particularly in the long term.
Conclusion
We describe pHi measurements in the mantle epithelial cells of C. gigas using an established microfluorimetric cell-imaging technique. The detected differences in pHi regulatory capacities are a first step in identifying the functional cellular pathways for acid–base homeostasis of these cells. The decreased capacities of mantle epithelial cells to recover from an induced cellular acidosis as a result of exposure to low Na+ and all three pharmacological inhibitors indicate that Na+-driven ion transport pathways and carbonic anhydrases are an important component of the pHi regulatory machinery in these cells. These findings are summarized in a first preliminary model of the pHi homeostasis machinery in mantle cells, highlighting the necessity for maintaining a Na+ gradient as driving force (NKA and NHE) and of CA in facilitating proton and bicarbonate generation (Supplementary Fig. 3). At present, several open questions remain regarding the physiology of molluscan calcification including the identification of bicarbonate transporters involved and the role of septate junctions in extracellular calcium transport.
Data availability
Data can be accessed through PANGAEA database (https://doi.org/10.1594/PANGAEA.920870).
References
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochem Moscow 65:757–765
Aguilera F, McDougall C, Degnan BM (2017) Co-option and de novo gene evolution underlie molluscan shell diversity. Mol Biol Evol 34:779–792
Awaji M (1991) Separation of outer epithelial cells from pearl oyster mantle by dispase digestion. In: Franser, M.J. Jr. (ed.) Proceedings of the 8th International Conference on Invertebrate and Fish Tissue Culture, June 16–20, 1991, Anaheim. Tissue Culture Association, Columbia, MD. pp 43–46
Banakou E, Dailianis S (2010) Involvement of Na+/H+ exchanger and respiratory burst enzymes NADPH oxidase and NO synthase, in Cd-induced lipid peroxidation and DNA damage in haemocytes of mussels. Comp Biochem Physiol C: Toxicol Pharmacol 152:346–352
Beckmann MJ (2011) Modulation of intracellular pH of hemocytes from the pacific oyster Crassotrea gigas to changes in extracellular pH. Master thesis, Alfred Wegener Institute, Bremerhaven, Germany
Boron WF (1977) Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol 233:C6I–C73
Bourgeois S, Bounoure L, Mouro-Chanteloup I, Colin Y, Brown D, Wagner CA (2018) The ammonia transporter RhCG modulates urinary acidification by interacting with the vacuolar proton-ATPases in renal intercalated cells. Kidney Int 93:390–402
Burton RF (1978) Intracellular buffering. Resp. Physiol 33:51–58
Cao-Pham AH, Hiong KC, Boo MV, Choo CYL, Pang CZ, Wong WP, Neo ML, Chew SF, Ip YK (2019) Molecular characterization cellular localization and light-enhanced expression of Beta-Na+/H+ Exchanger-like in the whitish inner mantle of the giant clam Tridacna squamosa denote its role in light-enhanced shell formation. Gene 695:101–112
Chew SF, Koh CZY, Hiong KC, Choo CYL, Wong WP, Neo ML, Ip YK (2019) Light-enhanced expression of carbonic anhydrase 4-like supports shell formation in the fluted giant clam Tridacna squamosa. Gene 683:101–112
De Wit P, Durland E, Ventura A, Langdon C (2018) Gene expression correlated with delay in shell formation in larval Pacific oysters (Crassostrea gigas) exposed to experimental ocean acidification provides insights into shell formation mechanisms. Mol Ecol 19:160
Dwyer JJ, Burnett LE (1996) Acid-Base Status of the Oyster Crassostrea virginica in Response to Air Exposure and to Infections by Perkinsus marinus. Biol Bull 190:139–147
Ellington WR (1983) The extent of intracellular acidification during anoxia in catch muscle of two bivalve molluscs. J Exp Zool 227:313–317
Ellington WR (1993) Studies of intracellular ph regulation in cardiac myocytes from the marine bivalve mollusk, Mercenaria Campechiensis. Biol Bull 184:209–215
Gong N, Li Q, Huang J, Fang Z, Zhang G, Xie L, Zhang R (2008) Culture of outer epithelial cells from mantle tissue to study shell matrix protein secretion for biomineralization. Cell Tiss Res 333:493–501
Herlitze I, Marie B, Marin F, Jackson DJ (2018) Molecular modularity and asymmetry of the molluscan mantle revealed by a gene expression atlas. GigaScience 7:1–15
Hiong KC, Cao-Pham AH, Choo CYL, Boo MV, Wong WP, Chew SF, Ip YK (2017) Light-dependent expression of a Na+/H+ exchanger 3-like transporter in the ctenidium of the giant clam, Tridacna squamosa, can be related to increased H+ excretion during light-enhanced calcification. Physiol Rep 5:e13209
Hu MY, Lee JR, Lin LY, Shih TH, Stumpp M, Lee MF, Hwang PP, Tseng YC (2013) Development in a naturally acidified environment: Na +/H + -exchanger 3-based proton secretion leads to CO2 tolerance in cephalopod embryos. Front Zool 10:51
Hu MY, Guh YJ, Stumpp M, Lee JR, Chen RD, Sung PH, Chen YC, Hwang PP, Tseng YC (2014) Branchial NH + 4-dependent acid-base transport mechanisms and energy metabolism of squid (Sepioteuthis lessoniana) affected by seawater acidification. Front Zool 11:55
Hu MY, Yan JJ, Peterson I, Himmerkus N, Bleich M, Stumpp M (2018) SLC4 family bicarbonate transporter is critical for intracellular pH regulation and biomineralization in sea urchin embryos. eLife. https://doi.org/10.7554/elife.36600
Ivanina AV, Falfushynska HI, Beniash E, Piontkivska H, Sokolova IM (2017) Biomineralization-related specialization of hemocytes and mantle tissues of the Pacific oyster Crassostrea gigas. J Exp Biol 220:3209–3221
Joubert C, Piquemal D, Marie B, Manchon L, Pierrat F, Zanella-Cléon I, Cochennec-Laureau N, Gueguen Y, Montagnani C (2010) Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization. BMC Genomics 11:613
Kaloyianni M, Stamatiou R, Dailianis S (2005) Zinc and 17 β-estradiol induce modifications in Na+/H+ exchanger and pyruvate kinase, activity, through protein kinase C in isolated mantle/gonad cells of Mytilus galloprovincialis. Comp Biochem Physiol C-Tox Pharm 141:257–266
Koh CZY, Hiong KC, Choo CYL, Boo MV, Wong WP, Chew SF, Neo ML, Ip YK (2018) Molecular characterization of a dual domain carbonic anhydrase from the ctenidium of the giant clam Tridacna squamosa and its expression levels after light exposure cellular localization and possible role in the uptake of exogenous inorganic carbon. Front Physiol 9:281
Laurent J, Venn A, Tambutté E, Ganot P, Allemand D, Tambutté S (2014) Regulation of intracellular pH in cnidarians: response to acidosis in Anemonia viridis. FEBS J 281:683–695
Li S, Huang J, Liu C, Liu Y, Zheng G, Xie L, Zhang R (2016) Interactive effects of seawater acidification and elevated temperature on the transcriptome and biomineralization in the pearl oyster Pinctada fucata. Environ Sci Technol 50:1157–1165
Marie B, Luquet G, Bédouet L, Milet C, Guichard N, Medakovic D, Marin F (2008) Nacre calcification in the freshwater mussel Unio pictorum: carbonic anhydrase activity and purification of a 95 kDa calcium-binding glycoprotein. ChemBioChem 9:2515–2523
Medakovic D (2000) Carbonic anhydrase activity and biomineralization process in embryos, larvae and adult blue mussels Mytilus edulis L. Helgoland Mar Res 54:1–6
Melzner F, Mark FC, Siebel BA, Tomanek L (2020) Ocean acidification and coastal marine invertebrates: tracking CO2 effects from seawater to the cell. Annu Rev Mar Sci 12:499–523
Michaelidis B, Haas D, Grieshaber MK (2005) Extracellular and intracellular acid-base status with regard to the energy metabolism in the oyster Crassostrea gigas during exposure to air. Physiol Biochem Zool 78:373–383
Miyamoto H, Miyoshi F, Kohno J (2005) The carbonic anhydrase domain protein nacrein is expressed in the epithelial cells of the mantle and acts as a negative regulator in calcification in the mollusc Pinctada fucata. Zool Sci 22:311–315
Mount AS, Wheeler AP, Paradkar RP, Snider D (2004) Hemocyte-mediated shell mineralization in the eastern oyster. Science 304:97–300
Neff JM (1972) Ultrastructural studies of periostracum formation in the hard-shelled clam Mercenaria mercenaria (L.). Tiss Cell 4:311–326
Potts WTW (1958) The inorganic and amino acid composition of some lamellibranch muscles. J Exp Biol 35:749–764
Ramesh K, Yarra T, Clark M, John U, Melzner F (2019) Expression of calcification-related ion transporters during blue mussel larval development. Ecol Evol 9:7157–7172
Sillanpaa K, Sundh H, Sundell KS (2018) Calcium transfer across the outer mantle epithelium in the Pacific oyster, Crassostrea gigas. Proc. Royal Soc B. https://doi.org/10.1098/rspb.2018.1676
Stapp LS, Parker LM, O’Connor WA, Bock C, Ross PM, Pörtner HO, Lannig G (2018) Sensitivity to ocean acidification differs between populations of the Sydney rock oyster: role of filtration and ion-regulatory capacities. Mar Environ Res 135:103–113
Stumpp M, Hu MY, Melzner F, Gutowska MA, Dorey N, Himmerkus N, Holtmann WC, Dupont ST, Thorndyke MC, Bleich M (2012) Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proc Natl Acad Sci USA 109:18192–18197
Thomas RC (1974) Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol 238:159–180
Tresguerres M (2014) sAC from aquatic organisms as a model to study the evolution of acid/base sensing. Biochim Biophys Acta 1842:2629–2635
Vilain JP, Rodeu JL, Gaillard S (1993) Fluorescent probe measurement of intracellular pH during meiosis reinitiation by ammonia in oocytes of the Mollusc, Patella Vulgata. Comp Biochem Physiol 104A:479–482
Wang X, Fan W, Xie L, Zhang R (2008) Molecular cloning and distribution of a plasma membrane calcium ATPase homolog from the pearl oyster Pinctada fucata, Tsinghua. Sci Technol 13:439–446
Wang X, Wang M, Jia Z, Wang H, Jiang S, Chen H, Wang L, Song L (2016) Ocean acidification stimulates alkali signal pathway: a bicarbonate sensing soluble adenylyl cyclase from oyster Crassostrea gigas mediates physiological changes induced by CO2 exposure. Aquat Toxicol 181:124–135
Wang X, Wang M, Jia Z, Qiu L, Wang L, Zhang A, Song L (2017) A carbonic anhydrase serves as an important acid-base regulator in pacific Oyster Crassostrea gigas Exposed to Elevated CO2: implication for physiological responses of mollusk to ocean acidification. Mar Biotechnol 19:22–35
Wiseman RW, Ellington WR (1989) Intracellular buffering in molluscan muscle: superfused muscle versus homogenates. Physiol Zool 62:541–558
Yu Z, Xie L, Lee S, Zhang R (2006) A novel carbonic anhydrase from the mantle of the pearl oyster (Pinctada fucata). Comp. Biochem Physiol B 143:190–194
Zange J, Grieshaber MK, Jans AWH (1990) The regulation of intracellular pH estimated by P-NMR spectroscopy in the anterior byssus retractor muscle of Mytilus edulis L. J Exp Biol 150:95–109
Zeebe RE, Wolf-Gladrow DA (2001) CO2 in seawater: equilibrium, kinetics, isotopes. In: Halpern D (ed) Elsevier oceanography series, vol 65. Elsevier, Amsterdam, p 346
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PWH, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Lošo T, Du Y, Sun X, Zhang S, Liu L, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, Du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinber CEW, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54
Zhao L, Schöne BR, Mertz-Kraus R, Yang F (2017a) Insights from sodium into the impacts of elevated pCO2 and temperature on bivalve shell formation. J Exp Mar Biol Ecol 486:148–154
Zhao L, Schöne BR, Mertz-Kraus R, Yang F (2017b) Sodium provides unique insights into transgenerational effects of ocean acidification on bivalve shell formation. Sci Total Environ 577:360–366
Zlatkin RL, Heuer RM (2019) Ocean acidification affects acid–base physiology and behaviour in a model invertebrate, the California sea hare (Aplysia californica). R Soc Open Sci. https://doi.org/10.1098/rsos.191041
Acknowledgements
Open access funding provided by University of Gothenburg. This study is funded by the European Union’s Seventh Framework Programme [FP7] ITN project ‘CACHE’ under REA Grant agreement #[605051]13. M. Hu is funded by the Emmy-Noether Program (403529967) of the erman Research Foundation (DFG).
Author information
Authors and Affiliations
Contributions
KR and FM designed the study. FM and MB contributed to reagents and analytical tools. KR, MYH, NH conducted experiments. KR analysed the data and wrote the manuscript. All authors contributed to manuscript revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Bernd Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramesh, K., Hu, M.Y., Melzner, F. et al. Intracellular pH regulation in mantle epithelial cells of the Pacific oyster, Crassostrea gigas. J Comp Physiol B 190, 691–700 (2020). https://doi.org/10.1007/s00360-020-01303-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01303-3