Abstract
The Valaisan-type armband (a specific type of bracelet) is a typical metallurgical production from the western area of Switzerland belonging to the classic phase of the Aare-Rhone group (BzA2a, ca. 2000–1800 BC). This investigation aims to (i) characterize the metal composition, (ii) reconstruct the thermomechanical treatments applied during the manufacturing process, and (iii) gather information on the possible exploitation of the local ores coupling metallography and chemical analysis. The results show that each armband is manufactured from a hammered sheet of copper-based alloy, containing either tin (up to 3.0 wt.%) or a combination of antimony, nickel, and silver. In several cases, it is assumed that minor elements are already part of the original ore, suggesting a conscious selection of copper veins. In other armbands, a direct addition of cassiterite (SnO2) to the copper matrix is hypothesized based on the material composition and features of the inclusions. Microstructural features are coherent with a procedure that combines mechanical deformation (with a total deformation degree between 70 and 76%), annealing, and quenching, coherently with more recent productions (Late Bronze Age). The analysis of inclusions, rarely performed during metallographic investigations, provides precious evidences on thermal treatments applied during the manufacturing process and shows that annealing was carried out at low temperatures.
Similar content being viewed by others
Introduction
The ways of development and spread of metallurgical practices in the Early Bronze Age (EBA) constitute an important issue in archaeometallurgy but sometimes the lack of physical evidences for this period often limit the investigations on early practices. As remains of ancient civilizations, copper-based objects are the only exploitable source of information in such sense. The abundance of copper objects found in the areas of Singen (Germany), Franzhausen and Gemeinlebarn (Austria), and in the central Valais region (Switzerland) represents an exception that could help gather information on (i) metallurgical practices, (ii) circulation of raw materials and finished products, and (iii) transfer of knowledge (Cattin et al. 2011). In this context, Valaisan-type armbands, literally translated from the French brassards (Cattin et al. 2011), found in the western area of Switzerland and belonging to the classic phase of the Aare-Rhone group (ca. 2000–1800 BC) are studied by the authors in order to answer some of the abovementioned questions.
Archaeological studies thoroughly describe the Rhone culture that extends between Eastern France and Western Switzerland along the Rhone River during the EBA (ca. 2200–1500 BC) (Cattin et al. 2011; David-Elbiali and David 2009; David-Elbiali 1998, 2000). Hafner (1995) defined the Aare-Rhone group according to the names of the two rivers that delimit this area. Hammered sheet objects (e.g., pins, diadems, and lunulae), typical of a specialized craftmanship (Strahm 1996), were mainly discovered in the so-called Blechkreis (that embraces the Western Switzerland and the Middle Danube) and testify a specific metalworking activity. According to such studies, the main archaeological issues concerned the emergence of bronze production and the development of different manufacturing techniques. In particular, ore exploitation, production, and circulation of raw materials (like copper or tin) over long distance are still under debate (Radivojević et al. 2018; Berger et al. 2018), and even if few evidences of copper smelting are present in this region, they are dated back to more recent periods (Late Bronze Age, LBA) (Gallay 2008). While previous analyses were performed on Valaisan armbands (atomic absorption spectroscopy (AAS), induced coupled plasma-optical emission spectroscopy (ICP-MS), electron probe micro-analyzer (EPMA) coupled with wavelength dispersion spectroscopy (WDS)), allowing the detection of trace elements (ppm) on chemically treated bulk samples (Junghans et al. 1960, 1968 & 1974; Pernicka 1984), recent studies were carried out to answer some questions, focusing on the routes of copper supply (Cattin 2008; Cattin et al. 2011; Artioli et al. 2016). Through chemical and lead isotope analysis, the authors suggested a correlation between the local manufacture of hammered sheets, characteristic of the Aare-Rhone group, and the copper supply chain, which can be usually provided either from recycling activities or from exogenous sources. However, the results obtained are still under discussion and further analyses are needed to provide robust answers to such an important issue. To fully address this lack of data, high-quality information can be obtained through metallurgical investigations by exploiting the ability of metallic materials to record its own thermomechanical history (Piccardo et al. 2017a, 2017b). This way, nondestructive (i.e., which do not alter the sample during analysis) and micro-invasive (i.e., which only require micrometric samples) procedures result in a good compromise between the quality of information and the object alteration (Pinasco et al. 2007; Piccardo et al. 1997). However, the use of nondestructive and noninvasive methods (e.g., p-XRF) is sometimes not enough to get sufficient information about the metallic core and the presence and distribution of phases and inclusions, when a patina of corrosion product covers the surface of the artifact (Fernandes et al. 2013; Nørgaard 2017). Metallographic studies, on the other hand, allow for the reconstruction of the thermomechanical history of materials by analyzing their microstructural features through the chemical characterization of the metallic matrix and inclusions (Piccardo et al. 2017a, 2017b).
The present article focuses on the metallographic characterization of a selected corpus of 18 Valaisan-type armbands coming from the western area of Switzerland and belonging to the Aare-Rhone group to obtain new evidences that will help in correlating the manufacturing process to the copper supply chain. This study especially aims to (i) characterize the metal composition (major and minor elements) in order to establish the relationship between the final product, raw materials, and anthropogenic addition (i.e., deliberate alloying), (ii) reconstruct the manufacturing process of the objects through the thermomechanical treatments observed, and (iii) gather information on the possibility of local ore exploitation.
Materials and methods
The armbands
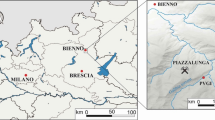
The corpus is composed of 18 Valaisan-type armbands over the 22 currently conserved in two different collections: the Musee Cantonal d’Histoire du Valais (MCA) from Sion (Switzerland) and the second at the Musee Cantonal d’Archeologie et d’Histoire (MCAH) from Lausanne (Switzerland) (see Table 1). The discovery sites are located in the central Valais (VS) region and in the Chablais part of the Vaud region (VD), respectively (Bocksberger 1964; Pászthory 1985) This area of about 100 km2 constitutes one of the main poles of the Rhone culture (Fig. 1). The corpus, previously catalogued and partially studied by David-Elbiali during her PhD thesis (David-Elbiali 2000), is in a quite good conservation state.
The armbands were discovered in burial contexts (David-Elbiali 2000), except for the armbands from the site of Leuk, which were part of a hoard. Identified as feminine ornaments, they are made of semi-circular sheets (Fig. 2), specific of classic phase sites of the Aar-Rhone group (Cattin et al. 2014), and decorated with deep-drawn motives surrounded by triangular and crosspieces incised lines. Square or circular holes are systematically present on each edge, suggesting a binding system made of organic materials (e.g., leather or tissue). Also, it is not to exclude that organic material was placed in contact with the skin as a further protection.
Since this typology was only found in sites from Western Switzerland (David-Elbiali and David 2009), these armbands can be considered a representative of the local material culture with very low possibilities of a non-local manufacturing process (Cattin et al. 2014). However, similar features were found in Lower Austria (Weinviertel) and in Moravia, at the southern border of the Unetice culture (the so-called Borotice-type) and interpreted as a direct influence of the southeastern neighbors, suggesting a transfer of knowledge (Schubert 1974; David-Elbiali 2000; David 2002).
Sampling and protocol of analysis
A careful macroscopic examination of the surface of the objects was carried out before the sampling procedure to evidence previous restorations, tool traces, mechanical stresses, critical zones (i.e., brittle or fragmented), and worn areas. The most representative areas were selected for sampling, in order to preserve the integrity of the objects without altering their readability. The small-size fragments (between 2- and 4-mm height) were mounted in epoxy resin and then suitably polished with diamond paste up to 0.25 μm. Chemical etching was also performed with a solution of FeCl3 (5 g) diluted in HCl (50 mL) and H2O (200 mL) on the samples in order to reveal their microstructural features. The protocol of analysis included (i) observations through light optical microscopy (LOM) using bright field (BF) and dark field (DF) contrast methods, (ii) observations through scanning electron microscopy (SEM) with backscattered (BSE) and secondary electron (SE) detectors as contrast methods, and (iii) quantitative analysis through energy-dispersive X-ray spectroscopy (EDS) sensitive to light elements. The EDS was previously calibrated on a cobalt standard. This procedure allowed to obtain reliable values for all elements with atomic weight higher than sodium (i.e., Z ≥ 11), while for lighter elements like oxygen, the analysis was considered semiquantitative. Amounts below 0.3 wt.% were also considered semiquantitative measurements and were evaluated only when the identification peaks were clearly visible in the acquisition spectrum. Trace elements cannot be detected with SEM-EDS since below the limit of detection (LOD) of the instrument.
The reported compositions of the objects correspond to the average of at least three measurements on non-corroded areas. All results were normalized and presented in average weight percent. A statistical procedure was also performed using the principal component analysis (PCA) in order to evidence correlations between the variables and clustering between samples. This multivariate data treatment is based on the reduction of the number of system variables by regrouping them in principal component (PC), which allows the definition of a new coordinate system in 2 dimensions. As a result, the original data matrix was decomposed into two smaller matrices: the loadings, which can be interpreted as the weights for each original variable in the PC computation, and the scores, which contain the original data in the newly rotated coordinate system. Also, a biplot was created with both the loading (variables) and score (samples) positions on the new coordinate system.
To estimate the deformation rate and the initial thickness of the object, an image analysis software (Fiji-ImageJ, version 1.49b) was used on × 100 LOM micrographs (Schindelin et al. 2012).
Results
Alloy composition and microstructure
According to the chemical concept, an alloy is any element other than a pure metal element (Tweney and Hughes 1949). From a technological point of view, this results in changes in mechanical, physical, and chemical properties or manufacturing/processing characteristics (Davis 2001). Thus, the concept of alloying is independent of a deliberate addition but rather refers to physical modifications of the base metal. In this study, the authors considered each detected element as an alloying element, either major or minor. Table 2 displays the results on the composition of the 18 samples acquired through the EDS measurements. For each armband, the alloying elements in the matrix (Sn, Sb, Ag, and Ni) never exceed 5.0 wt.%.
Figure 3 displays the biplot obtained from the PCA where three main compositional clusters are distinguished:
Group A – Nine objects made of raw non-alloyed copper (Cu ≥ 99.5 wt.%) or with low tin amounts (between 0.7 and 1.3 wt.%) with evidences of silver-rich precipitates and tin oxide inclusions (SnO2). These objects come from Leuk (2 armbands out of 2), Sierre (2/5), Ayent (3/5), Ollon (1/5), and Conthey (1/1) (Fig. 4A and D).
Microstructures from the three groups identified. LOM-BF micrographs of (A) sample 17 (MCA VS 40592b from Leuk VS Foret de Guttet), (B) sample 1 (MCAH VD 20 from Ollon VD Derriere la Roche), and (C) sample 3 (MCAH VD 27 from Ollon VD Verschiez). SEM-BSE micrographs of (D) sample 17, (E) sample 1, and (F) sample 3
Group B - Five objects presenting Sn as principal alloying element with concentrations between 1.9 and 3.2 wt.% (Fig. 4B and E) from Ayent (2/5) and Ollon (3/5).
Group C - Four objects containing a combination of Sb, Ag, and Ni from Ollon (1/5) and Sierre (3/5) (Fig. 4C and F). Tin has not been detected for these armbands or in very low amount.
Armbands containing enough alloying elements (samples 1 to 6 from Ollon and samples 6 to 9 from Ayent) are characterized by the residual heterogeneity of the solid solution as evidenced by Fig. 4E, in which grayscale variations represent changes in composition. This microsegregation corresponds to a concentration gradient between the core of the primary crystal (dendrite) and its edges, directly related to a combination of casting process parameters (e.g., cooling rate) and mechanical deformation of the matrix (e.g., cold deformation and annealing) (Smallman and Ngan 2007). The observation on etched surfaces (Fig. 4A, B, and C) showed regular polygonal grains of relatively small size (micrometrics), typical of recrystallized microstructures resulting from a combination of mechanical deformation and annealing at intermediate temperatures (below 600 °C).
Inclusions
Various inclusions are visible in the metallic matrix (Fig. 5) and are identified as oxides, sulfides, or precipitates from the smelting procedure (Table 3). They are preferably located at the grain boundaries due to the segregation phenomena occurring during the molten-to-solid transition. Further elements (S, As, Ni, Co) are detected inside the inclusions, as precipitates, or in the internally developed corrosion layer.
Types of inclusions detected by SEM-BSE. (A) SnO2 on sample 7 (MCA VS 742 from Ayent VS Les Places), (B) Cu2SexS1-x inclusion on sample 4 (MCAH VD 28 from Ollon VD Verschiez), (C) Ag and Sb inclusions on sample 11 MCA VS 750 from Sierre VS Muraz), and (D) Sb-rich precipitates on sample 13 MCA VS 752 from Sierre VS Muraz)
The most common inclusions in the whole set of armbands are white-rounded tin oxides (cassiterite, SnO2) of various dimensions. They are present in the metallic matrix as well as in the patina (Fig. 5A). Elongated inclusions (Fig. 5B) composed of copper sulfide with selenium (Cu2SexS1-x) are present in a wide number of samples. No iron has been detected. A composite nodule, found in sample 3 from Ollon, corresponds to an inclusion of partially smelted ore (Fig. 6). The dark inner part of the nodule is rich in nickel (Ni) (between 15 and 23 wt.%) and cobalt (Co) (between 32 and 40 wt.%), with lower amount of tin (Sn), antimony (Sb), and silver (Ag). The brighter area surrounding this core corresponds to the partial diffusion of Sn and Sb into the copper matrix.
Further inclusions are concentrated in the intergranular corrosion layers. As previously discussed elsewhere (Ghiara 2016), the corrosion process is selective and depends on the cation’s diffusivity in the oxidizing medium (e.g., the soil): while copper tends to diffuse into the soil, the concentration of other elements in the patina increases. Figure 5C represents a clear example of this phenomenon. This way, the intergranular oxidation process reveals the presence of phases and elements whose concentration ratio is different from the one of the matrixes.
The white phases in the corroded areas (Fig. 5C) are metallic Ag with a volume percentage higher than the one characterizing the microstructure. This could be explained by a selective corrosion mechanism involving the metallic matrix, and the more noble element is left uncorroded (Shreir et al. 1963). As a matter of fact, Ag is detected in 11 out of 18 armbands of the corpus as a metallic phase, mainly visible in the corrosion layers. Under 400 °C, this element is scarcely soluble in the copper matrix and can generate either rounded precipitates at the grain boundary during the cooling phase after casting or needle-like precipitates inside the grains if formed during a thermal treatment below 400 °C (e.g., annealing). In 6 armbands, this element is combined with the Sb inclusions, and in two others from Sierre, it is associated with traces of As (Table 2).
A similar microstructural feature has been observed in sample 13 from Sierre showing Sb-rich precipitates (white acicular micro-crystals) at the grain boundaries and in the core of the solid solution (Fig. 5D). This corresponds to a typical precipitation occurring in hypoeutectic Cu-Sb alloys, when the supersaturated α-solid solution (previously obtained by fast cooling rates, or quenching) is annealed at a temperature below the eutectic temperature (e.g., 645 °C).
Sulfide inclusions present the peculiarity to deform with the bulk when submitted to mechanical stress, changing shape from rounded (i.e., typical of as-cast microstructures) to elongated along the direction of deformation, and then to keep such a shape even after recrystallization annealing. The shape factor of the inclusions (sf) can be computed by calculating their width/length ratio and by correlating it to the stress absorbed by the material (Piccardo and Pernot 1997; Mödlinger and Piccardo 2013). According to such studies, the deformation applied, resulting in a reduction of thickness (D%), is described as:
where thickend is final thickness of the metal sheet and thick0 the initial thickness of the metal plate after casting and before any shaping process is applied. The equation was then further implemented correlating the sf of the inclusions to the deformation applied:
Considering a statistical population of sulfides in one single sample, it is possible to calculate the order of magnitude of the deformation applied during the whole shaping process, i.e., from the as-cast state to the final object. Table 4 displays the deformation rates of three armbands containing enough sulfides to have a statistical reliability. Combining the metal plate thickness with the deformation ratio, the initial thickness of the as-cast draft can be estimated. This value is only an approximation of the real thick0 because of the underestimation related to the unknown amount of metal loss due to oxidation (e.g., during the annealing steps) and to surface finishing. The results are coherent with previous studies performed on findings from later periods like the LBA (Pernot and Lehoërff 2003).
Discussion
Alloying process and sources of exploitation
Alloying elements in archaeological artifacts made of copper might have two origins: (i) a natural occurrence in the copper-rich ore and the resulting diffusion during the smelting process; (ii) a deliberate addition by the metalworkers. However, a deliberate choice must be considered either way, as related to the selection of the ore, the mine, or the supplier. Copper mines containing tin-rich minerals are widely known (Niederschlag et al. 2003; Höppner et al. 2005; Artioli et al. 2009; Schreiner 2007; Frotzscher 2012; Delibes de Castro et al. 1998), and when tin is detected in the metallic matrix below 1.5 wt.%, it is usually accepted as a natural occurrence coming from the smelting procedure. On the other hand, an amount greater or equal to 1.5 wt.% can be considered an anthropological addition (Pernicka 1999). In this collection, 9 armbands over 18 can be classified this way (Table 2). From a preliminary study, the systematic presence of Sn oxides in the microstructure of the armbands could suggest a co-smelting process of both copper-rich and tin-rich ores, but the different smelting parameters of these elements make this process very unlikely. A direct addition of Sn as cassiterite or a partially reduced Sn (due to a previous smelting) can be assumed. The direct use of SnO2 for the alloying procedure opens an important issue because of the absence of known cassiterite or tin-bearing mines exploited in Western Switzerland during the EBA. At the current stage of the archaeological knowledge, only direct or indirect long-distance communication and material circulation with mines from Cornwall, Britain (Penhallurick 1986; Budd et al. 1992; Valera and Valera 2003; Haustein et al. 2010), or Erzgebirge, Germany (Niederschlag et al. 2003; Penhallurick 1986; Valera and Valera 2003; Schreiner 2007; Frotzscher 2012), can be hypothesized. Even if Sn mines in Tuscany have been identified for Etruscan metallurgy (Benvenuti et al. 2003), no exploitation evidences during the EBA have been found yet.
The detection of Ag, Ni, Sb, As, Zn, Pb, Co, and Sn as traces or inclusions (i.e., Ag and Sb/As) in the samples are related to the alloy production process and in particular to the chemical nature of the ore used for the copper extraction (Dupouy 1998; Ixer and Budd 1998). The classification of both metallic and nonmetallic impurity elements was used to provide information on the technological transitions of copper metallurgy, in particular for Fe, S, As, and Sb (Rychner and Kläntschi 1995; Tylecote 1977).
The so-called chalcolithic copper smelting model classically describes the early copper extraction processes, from the Late Neolitic to the first phases of the EBA, as a reduction of oxidized minerals like carbonates (malachite, azurite) or oxides (cuprite, tenorite). The emergence of more sophisticated processes for the copper sulfide smelting, like fahlerz, and later mixed copper and iron sulfides, appeared in more recent periods (Bourgarit 2007). As a support of this hypothesis, artifacts from the EBA often feature low sulfide amounts in the metallic matrix (Tylecote 2002). However, the early use of sulfide-based copper ores was also attested for several EBA-dated European sites, where the smelting was performed from mixed sulfide and oxidized minerals (Bourgarit and Mille 2001; Jovanovich and Ottaway 1976). In support of this hypothesis, experimental archaeology confirmed the possibility of direct reduction of some oxide-sulfide minerals (Timberlake 2007; Rostocker et al. 1989).
In the present study, two hypotheses can be formulated as related to the ores exploited for the production of the armbands. On the one hand, copper oxides like carbonates might have been used, which would be coherent with the low S and Fe amounts (Tylecote et al. 1977) and the large number of other elements previously listed (Delibes de Castro et al. 1998; Rychner and Kläntschi 1995; Rychner and Stos-Gale 1998). Furthermore, the detection of As in two out of 18 armbands can be explained either by the absence of such an element in the original ore or, more probably, by its evaporation during the smelting process (Mödlinger et al. 2017; Seeliger et al. 1985). On the other hand, the minerals used could be sulfides, particularly the system tennantite-tetrahedrite (Cu12Sb4S13) where Cu can be partially substituted by Ag, Zn, or Fe (Ixer and Budd 1998). This could justify the particularly high amount of Sb over As. However, more analyses have to be performed to fully understand this specific aspect of metal production. What can be drawn for these data is that the clear absence of iron in sulfide inclusions allows for the exclusion of ores that exploit chalcopyrite as a copper source. Furthermore, the partially smelted inclusions of sample 3 from Ollon may confirm the common mineralogical origin of Ni and Co, also suggesting that for 7 armbands (one from Ollon, two from Ayent, three from Sierre, and one from Leuk), copper could have been provided from minerals associated with Co-Ni arsenides (Staude et al. 2010). Ni inclusions detected systematically with Sb in 3 armbands (samples 9, 10, 17) could give a further indication of a specific ore source. No correlation can be suggested for the other objects.
According to such observations, three main clusters of objects can be suggested:
Natural copper alloys produced by the smelting of copper-rich ores, either oxidized or sulfide-based or a mix of the two types (most of them without sulfur): 7 objects (sample 3 from Ollon, samples 11, 12, 13, and 15 from Sierre and samples 17 and 18 from Leuk).
Natural tin-copper alloys, where tin is both present as micro-alloying element (< 1.5 wt.%) and as part of oxide inclusion: 5 objects (samples 6, 8, and 10 from Ayent, sample 14 from Sierre, and sample 16 from Conthey). In such a case, Sn most probably derives from the exploited minerals.
Anthropogenic tin-copper alloys, where tin is present in both the solid solution (≥ 1 wt.%) and as SnO2 inclusions, for which the higher tin amounts suggest a conscious post-smelting addition. These objects are usually also characterized by the presence of Cu sulfides. Such inclusions are rich in Se and suggest the exploitation of deeper veins: 6 objects (samples 1, 2, 4, and 5 from Ollon and samples 7 and 9 from Ayent).
If Sn, Sb, As, or even Ag are found as alloying elements together with residual copper or as inclusions (in the form of oxides), it is reasonable to make a direct connection with the original ores. From previous researches (Cattin 2008; Cattin et al. 2011), it is suggested that the exploitation of copper is derived from local ores, even if the distinction would require further analyses (Fig. 7A). Likewise, if the presence of tin is not a deliberate addition but present as micro-alloying element, further hypotheses could be made. In this period, ores containing both Cu and Sn minerals (Fig. 7B) can be found on the Austrian side of the European Alps (Niederschlag et al. 2003; Höppner et al. 2005), in Tuscany (Artioli et al. 2009; Benvenuti et al. 2003), in Erzgebirge, between Germany and Czech Republic (Niederschlag et al. 2003; Penhallurick 1986; Valera and Valera 2003), and in Central Germany (Frotzscher 2012). On the other hand, if SnO2 is deliberately added as source of tin, as it appears from the analyses, it opens new questions and fields of investigations. The exploitation of SnO2 as a Sn source is interesting and, besides being already explored in past researches (Radivojević et al. 2018; Berger et al. 2018; Vernet et al. 2019), is an important improvement in the alloy production. The presence of oxides in the metal matrix might be interpreted as the evidence of uncontrolled conditions during the manufacturing process, connected to a poor production of CO typical of non-aerated conditions, which can be coherent with the early copper smelting processes, carried out under relatively low temperatures and/or poorly reducing conditions (Bourgarit 2007). However, it is not possible to define with certainty the quantity and volume percentage of SnO2 inside an object, not even with chemical calculations that could indicate the amount of SnO2 originally added. The reason is connected to possible post-production processes such as re-melting (e.g., from ingot to object) and recycling (i.e., re-use and mix of alloys) that can affect the metallurgical features by reducing the size and volume fraction of the oxides without modifying the copper sulfides (Vernet et al. 2019). To our knowledge, no studies indicating re-melting are carried out in this region and for this specific period, but that does not mean that the procedure was not performed at all (Cattin 2008). This hypothesis could be confirmed only by the concomitant analysis of possible slags and/or crucibles, as demonstrated by Erb-Satullo (2015) in a different context, but this information for this context is, to our knowledge, still missing or currently unpublished. In the end, the absence of experimental data on this topic makes any further conclusion impossible, and a new field of experimental research is now open.
Shaping process
The armbands are recrystallized bronzes with regular polygonal grains, characteristic of the shaping sequence: cold hammering and recrystallization annealing sessions applied on an as-cast flat ingot. Mechanical twins and slip bands also indicate that some post-annealing cold deformation was carried out. The narrow range of composition makes these alloys very similar from the thermomechanical point of view. They are characterized by good shape properties, deformability, plasticity, resilience, and toughness. The same thermal treatments (e.g., annealing) can be successfully applied and the few differences in color (mainly for those armbands made of unalloyed copper) should become unnoticeable once the finished surface suffers from natural tarnishing (Fang and McDonnell 2011).
Through measurements of sulfide dimensions, the total thickness reduction performed during cold hammering stages is estimated between around 70 and 76 % (Table 4). On the one hand, the recrystallized microstructures confirm that annealing was carried out after many steps of mechanical deformation at a temperature high enough to recover the deformability of the metal. On the other hand, the presence of residual heterogeneity in the solid solution shows that this annealing was not enough in terms of temperature and/or time to homogenize the solid solution. Since the annealing temperatures for the complete recrystallization of tin bronzes are often considered in the range 500–600 °C (Smallman and Ngan 2007), it can be assumed that for this corpus no homogenizing annealing was performed before the mechanical deformation. Antimony-rich inclusions of sample 13 from Sierre could help identify the range of annealing temperatures for the corresponding armband. For the Sb amount detected in the alloy (1.7 wt.%), the Cu-Sb system phase diagram indicates that the annealing temperatures are between 310 and 360 °C (Ixer and Budd 1998). The presence of such insoluble inclusion shows that annealing was performed at a lower temperature than 360 °C, followed by a quenching stage. This is coherent with the presence of Ag precipitates in sample 11 from Sierre, which are insoluble in a copper matrix under 400 °C.
These low annealing temperatures (T < 360 °C) can be easily reached by using a plain wood fire. This would coincide with the preferential use of wood rather than charcoal to feed the fires during the annealing operations. These technical choices allow for an economy of fuel, time, and energy by keeping the charcoal for energy-consuming activities as smelting and casting. These evidences can be associated with both chronological and geographical aspects, translating an evolution of the methods during the 200 years of the Aar-Rhone group classical period, or with the differences between the craftsmanship related to each archaeological site or artifact.
Conclusions
Eighteen Valaisan-type armbands dated back to the EBA and belonging to the Aar-Rhone culture were investigated by a multi-technical approach with the aim to contribute to the identification of the sources of the raw materials and the reconstruction of the manufacturing process. The collection of data on the composition of the alloy and the nature of the inclusions, together with the observation of their microstructures, allowed to assess that armbands are made of a copper-based alloy undergoing a similar shaping process: alloy preparation by melting process of raw copper with or without the addition of alloying ingredients; casting of an ingot which might have been flat, with a thickness of ca. 1 mm; and application of several stages of cold hammering and annealing to reach a final thickness reduction between 70 and 76%.
Furthermore, the residual heterogeneity of the solid solution and the presence of Sb- or Ag-rich precipitates gives also information on the range of temperatures reached during the annealing treatments: between 300 and 360 °C, high enough to recrystallize a cold-hardened alloy but not enough to homogenize the solid solution. This can be due to multiple factors as technical evolution, availability of the resources, economy of time, and fuel. By the quality of the final product, it is evident that the metal craft technology is mature and well consolidated and can be compared to more recent productions, typical of LBA.
Important information could be gathered also on the alloying process, since three groups of objects could be identified: (i) natural alloys with and without Sn, (ii) anthropogenic alloys with Sn higher than the 1.9 wt.%, and (iii) alloys with Sb, Ag, and Ni as alloying elements. The first two sets of samples showed the presence of SnO2, evidencing cassiterite as the main alloying ingredient for the alloy production. The characterization of the metallic matrix thus highlighted the distinction of copper base alloys between “naturally” and “voluntarily” alloyed. The careful understanding of the information kept in the metal enriches the research on material provenance and distribution and knowledge “routes” across cultures and regions. Moreover, the insights on the consciousness on the materials’ and minerals’ properties contribute to the discussion on the choice of sources selection.
The partially reduced inclusions found in most of the objects testify a small number of casting after the smelting process and a possible direct link with the original ores. These outcomes would work synergistically with well-established trace analyses and lead isotope ratio studies in the perspective of identifying the actual SnO2 sources as well as verifying the introduction of a primitive bronze production in such an area.
References
Artioli G, Angelini I, Burger E, Bourgarit D (2009) Petrographic and chemical investigation of the earliest copper smelting slags in Italy: towards a reconstruction of the beginning of copper metallurgy. In: Proc. 2nd Intern. Conference “Archaeometallurgy in Europe 2007”, Aquileia, 17-21 June. Associazione Italiana di Metallurgia, Milano, pp. 1 – 9
Artioli G, Angelini I, Nimis P, Villa IM (2016) A lead-isotope database of copper ores from the southeastern Alps: a tool for the investigation of prehistoric copper metallurgy. J Archaeol Sci 75:27–39
Benvenuti M, Chiarantini L, Norfini A, Casini A, Guideri S, Tanelli G (2003) The Etruscan tin: a preliminary contribution from researches at Monte Valerio and Baratti-Populonia (Southern Tuscany, Italy). In: Giumlia-Mair A, Lo Schiavo F (eds) The Problem of Early Tin. Archeopress, Oxford, pp 55–66
Berger D, Figueiredo E, Brugmann G, Pernicka E (2018) Tin isotope fractionation during experimental cassiterite smelting and its implication for tracing the tin sources of prehistoric metal artefacts. J Archaeol Sci 92:73–86
Bocksberger OJ (1964) Age du Bronze en Valais et dans le Chablais vaudois. Dissertation, Université de Lausanne, Switzerland.
Bourgarit D (2007) Chalcolithic copper smelting. In: La Niece S, Hook D, Craddock P (eds) Metals and Mines, Studies in Archaeometallurgy. Archetype Publications in association with the British Museum, London, pp 3–14
Bourgarit D, Mille B (2001) La transformation en métal de minerais de cuivre à base de sulfures: et pourquoi pas dès le Chalcolithique. Revue d'Archéométrie 25:145–155
Budd P, Gale D, Pollard AM, Thomas RG, Williams PA (1992) The early development of metallurgy in the British Isles. Antiquity 66:677–686
Cattin F (2008) Modalités d’approvisionnement et modalités de consommation du cuivre dans les Alpes au 3ème millénaire avant notre ère : apport des analyses métalliques à la connaissance des peuplements du Néolithique final, du Campaniforme et du Bronze ancien. Dissertation, Université de Genève, Switzerland
Cattin F, Guénette-Beck B, Curdy P, Meisser N, Ansermet S, Hofmann B, Kündig R, Hubert V, Wörle M, Hametner K, Günther D, Wichser A, Ulrich A, Villa IM, Besse M (2011) Provenance of Early Bronze Age metal artefacts in Western Switzerland using elemental and lead isotopic compositions and their possible relation with copper minerals of the nearby Valais. J Archaeol Sci 38:1221–1233
Cattin F, Curdy P, Guénette-Beck B, Wichser A, Ulrich A, Hubert V, Hunger K, Wörle M, Hametner K, Günther D, Chateau-Smith C, Villa IM, Besse M (2014) The copper-based artefacts from Sion/Petit-Chasseur (Valais, Switzerland) during the Late Neolithic, the Bell Beaker period and the Early Bronze Age (3200–1550 BC). In : Besse M (ed) Around the Petit-Chasseur site in Sion (Valais, Switzerland) and new approaches to the Bell Beaker culture, Proceedings of the international conference held at Sion (Switzerland), october 27th-30th, 2011
David, W (2002) Studien zur Ornamentik und Datierung der bronzezeitlichen Depotfundgruppe Hajdusamson-Apa-Ighiel-Zajta. Altip S.A., Alba Iulia.
David-Elbiali M (1998) Relations Suisse occidentale/Europe centrale au début du IIe millénaire av. J.-C. et révision de la chronologie relative et absolue de la culture du Rhône. Bull. D’études Préhist. Archéol. Alpines (Aoste) 9:105–116
David-Elbiali M (2000) La Suisse occidentale au IIè millénaire av. J.-C. : chronologie, culture, intégration européenne. Cahiers d’archéologie romande, Lausanne, pp 229-231.
David-Elbiali M, David W (2009) À la suite de Jacques-Pierre Millotte, l'actualité des recherches en typologie sur l'âge du Bronze. Le Bronze ancien et le début du Bronze moyen : cadre chronologique et liens culturels entre l'Europe nord-alpine occidentale, le monde danubien et l'Italie du Nord. In: Richard A, Barral P, Daubigney A, Kaenel G, Mordant C, Pinningre JF (eds) L’isthme européen Rhin-Saône-Rhône dans la Protohistoire. Approches nouvelles en hommage à Jacques-Pierre Millotte. Presses universitaires de Franche-Comté, Besançon, pp 311-340
Davis JR (ed) (2001) Alloying: understanding the basics, ASM International. Materials Park, Ohio
Delibes de Castro G, Fernandez Manzano J, Herran Martinez JI (1998) La métallurgie de l’age du cuivre dans le nord du plateau espagnol: caractéristiques des coulées et systèmes de production. In: Mordant C, Pernot M, Rychner V (eds) L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère, Les analyses de composition du métal: leur apport à l’archéologie de l’Age du Bronze. CTHS EDITION, Bordeaux, pp 63–80
Dupouy JM (1998) Réflexions sur l’intérêt des proportions relatives des teneurs en impuretés métalliques et de l’analyse des impuretés non métalliques pour la connaissance de la fabrication des bronzes. In: Mordant C, Pernot M, Rychner V (eds) L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère, Les analyses de composition du métal: leur apport à l’archéologie de l’Age du Bronze. CTHS EDITION, Bordeaux, pp 41–52
Fang JL, McDonnell G (2011) The color of copper alloys. Hist. Metall. 45:52–61
Fernandes R, van Os BJ, Huisman HD (2013) The use of handheld XRF for investigating the composition and corrosion of Roman copper-alloyed artefacts. Herit Sci 1:30. https://doi.org/10.1186/2050-7445-1-30
Frotzscher M (2012) Geochemische Charakterisierung von mitteleuropäischen Kupfervorkommen zur Herkunftsbestimmung des Kupfers von der Himmelsscheibe von Nebra. Forschungsberichte des Landesmuseums für Vorgeschichte Halle. Himmelscheibe von Nebra. "Der Aufbruch zu neuen Horizonten", DFG-Projekt FOR 550" 1, Halle an der Saale 2012
Gallay A (ed) (2008) Des Alpes au Léman: Images de la préhistoire. Infolio, Gollion
Ghiara G (2016). Long-term alteration mechanisms on copper-based alloys. The alloy patina environment system and their interactions. Dissertation, Università degli Studi di Genova, Italy
Hafner A (1995) Die Frühe Bronzezeit in der Westschweiz: Funde und Befunde aus Siedlungen, Gräbern und Horten der entwickelten Frühbronzezeit. Staatlicher Lehrmittelverlag, Bern, pp 1–277
Haustein M, Gillis C, Pernicka E (2010) Tin isotopy – a new method for solving old questions. Archaeometry 52(5):816–832
Höppner B, Bartelheim M, Huisjmans M, Krauss R, Martinek KP, Pernicka E, Schwab R (2005) Prehistoric copper production in the Inn Valley (Austria), and the earliest copper in central Europe. Archaeometry 47(2):293–315
Ixer RA, Budd P (1998) The mineralogy of Bronze Age copper ores from the British Isles: implications for the composition of early metalwork. Oxf J Archaeol 17(1):15–41
Jovanovich B, Ottaway BS (1976) Copper metallurgy and mining in the Vinca group. Antiquity 50:104–113
Junghans S, Sangmeister E, Schröder M (1960) SAM I: Metallanalysen kupferzeitlicher und frühbronzezeitlicher Bodenfunde aus Europa. Mann, Berlin
Junghans S, Sangmeister E, Schröder M (1968 & 1974) SAM II: Kupfer und Bronze in der frühen Metallzeit Europas. Vol. 1 – 4. Mann, Berlin
Mödlinger M, Piccardo P (2013) Manufacture of Eastern European decorative tin-bronze discs from twelfth century BC. Archaeol Anthropol Sci 5:299–309
Mödlinger M, De Oro CR, Haubner R (2017) Arsenic loss during metallurgical processing of arsenical bronze. Archaeol Anthropol Sci 11(1):133–140
Niederschlag E, Pernicka E, Seifert TH, Bartelheim M (2003) The determination of lead isotope ratios by multiple collector ICP-MS: a case study of early Bronze Age artifacts and their possible relation with ore deposits of the Erzgebirge. Archaeometry 45(1):61–100
Nørgaard H (2017) Portable XRF on prehistoric bronze artefacts: limitations and use for the detection of Bronze Age metal workshops. Open Archaeol 3:101–122
Pászthory K (1985) Der bronzezeitliche Arm- und Beinschmuck in der Schweiz. Prähistorische Bronzefunde Abteilung X, 3, München.
Penhallurick RD (1986) Tin in antiquity: its mining and trade throughout the ancient world with particular reference to Cornwall. Institute of Metals, London
Pernicka E (1984) Instrumentelle Multi-Elementanalyse archäologischer Kupfer- und Bronzeartefakte. Ein Methodenvergleich. Jahrb. Röm. –Germ. Zentralmus. 31:517–531
Pernicka E (1999) Trace element fingerprinting of ancient copper: a guide to technology or provenance? In: Young SMM, Pollard AM, Budd P, Ixer RA (eds) Metals in Antiquity. Archaeopress, Oxford, pp 163–171
Pernot M, Lehoërff A (2003) Battre le bronze il y a trois mille ans en Europe occidentale. Technè 18:43–48
Piccardo P, Pernot M (1997) Studio analitico strutturale di alcuni vasi celtici in bronzo. La metallurgia italiana 11:43–52
Piccardo P, Ghiara G, Bongiorno V, Vernet J, Spotorno R (2017a) SEM on metal archaeological findings: case studies. Microscopie 27(1):59–61
Piccardo P, Vernet J, Ghiara G (2017b) Mise en oeuvre des alliage cuivreux: faire parler le métal grâce à la science des matériaux. In: Pernot M (ed) Quatre mille ans d'histoire du cuivre: Fragments d'une suite de rebonds. THEA éditeur, Bordeaux, pp 41–60
Pinasco MR, Ienco MG, Piccardo P, Pellati G, Stagno E (2007) Metallographic approach to the investigation of metallic archaeological objects. Annali di Chimica 97:553–574
Radivojević M, Roberts BW, Pernicka E, Stos-Gale Z, Martinón-Torres M, Rehren T, Bray P, Brandherm D, Ling J, Mei J, Vandkilde H, Kristiansen K, Shennan SJ, Broodbank C (2018) The provenance, use, and circulation of metals in the European Bronze Age: the state of debate. J Archaeol Res 27(2):1–55
Rostocker W, Pigott VC, Dvorak JR (1989) Direct reduction to copper metal by oxide-sulphide mineral interaction. Archaeomaterials 3:69–87
Rychner V, Kläntschi N (1995) Arsenic, nickel et antimoine: une approche de la métallurgie du Bronze moyen et final en Suisse par l’analyse spectrométrique. Tome II. Cahiers d’archéologie romande, 64, Cahiers d'archéologie romande, Lausanne.
Rychner V, Stos-Gale Z (1998) Compositions chimiques et isotopes du plomb: la production métallique de l’Age du Bronze moyen et final en Suisse. In: Mordant C, Pernot M, Rychner V (eds) L’Atelier du bronzier en Europe du XXe au VIIIe siècle avant notre ère, Les analyses de composition du métal: leur apport à l’archéologie de l’Age du Bronze. CTHS EDITION, Bordeaux, pp 153–174
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis, Nat. Methods 9(7):676–682
Schreiner M (2007) Erzlagerstätten im Hrontal, Slowakei. Genese und prähistorische Nutzung. Forschungen zur Archäometrie und Altertumswissenschaft 3, Rahden, VML Verlag Marie Leidorf.
Schubert E (1974) Studien zur frühen Bronzezeit an der mittleren Donau. Walter de Gruyter Verlag, Berlin
Seeliger TC, Pernicka E, Wagner GA, Begemann F, Schmitt-Strecker SF, Eibner C, Öztunali Ö, Baranyi I (1985) Archaeometallurgische Untersuchungen in Nord- und Ostanatolien. Jahrb Römisch-Germanisch Zentralmuseums Mainz 32:597–659
Shreir LL, Jarman RA, Burstein GT (eds) (1963) Corrosion. Butterworth-Heinemann, Oxford
Smallman RE, Ngan AHW (2007) Physical metallurgy and advanced materials (7th edition). Butterworth-Heinemann, Oxford
Staude S, Mordhorst T, Neumann R, Prebeck W, Markl G (2010) Compositional variation of the tennantite-tetrahedrite solid solution series in the Schwarzwald ore district (SW Germany): the role of mineralization processes and fluid source, Mineral. Magazine 74(2):309–339
Strahm C (1996) Le Bronze ancient dans le sud-ouest de l’Allemagne. In: Mordant C, Gaiffe O (eds) 117 Congr. Nat. Soc. Hist. Scient (Clermont-Ferrand 1992). C.T.H.S., Paris, pp 251-268
Timberlake S (2007) The use of experimental archaeology/archaeometallurgy for the understanding and reconstruction of Early Bronze Age mining and smelting technologies. In S La Niece, D Hook, P Craddock (eds.) Metals and Mines, Studies in Archaeometallurgy, Archetype Publications in association with the British Museum, pp. 27 – 36
Tweney CF, Hughes LEC (1949) Chambers’s Technical Dictionary, W & R Chambers LTD, Edinburgh.
Tylecote RF (1977) Partitioning of trace elements between the ores, fluxes, slags and metal during the smelting of copper. J Archaeol Sci 4:305–355
Tylecote RF (2002) A history of metallurgy (2nd edition). Maney Publishing, Oxford
Valera RG, Valera PG (2003) Tin in the Mediterranean area: history and geology. In: Giumlia-Mair A, Lo Schiavo F (eds) The Problem of Early Tin. Archeopress, Oxford, pp 3–14
Vernet J, Ghiara G, Piccardo P (2019) Are tin oxides inclusions in early archaeological bronzes a marker of metal recycling? J Archaeol Sci: Reports 24:655–662
Acknowledgments
The authors would like to thank Florence Cattin for her wise support and priceless collaboration during this research, the restoration departments of the Musee d’Histoire du Valais from Sion (Switzerland) and the Musee Cantonal d’Archeologie et d’Histoire from Lausanne (Switzerland) for their hospitality during the sampling session.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piccardo, P., Vernet, J., Voland, G. et al. Metallographic investigation of Early Bronze Age armbands from Western Switzerland (ca. 2200–1500 BC): new highlights about early manufacturing processes. Archaeol Anthropol Sci 12, 215 (2020). https://doi.org/10.1007/s12520-020-01178-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-020-01178-z