Abstract
Nanoparticle Na3V2(PO4)3/C materials with different polyacrylonitrile contents can be successfully synthesized using precipitation method. As an energy storage material used in the production of lithium batteries which is also compared with pure of that, the Na3V2(PO4)3/C not only has a larger capacity, but also has a very high cycle stability. In particular, the initial discharge capacities of the Na3V2(PO4)3/C sample annealed at 750°C containing 10 wt% of polyacrylonitrile are 130.9 and 122.2 mAh g−1 at current densities of 0.5 and 1 C, respectively; the corresponding coulomb efficiencies are 97.3 and 99.5%. It is also worth noting that the capacity retention can reach as high as 92.7% for 500 cycles at 1 C.
Similar content being viewed by others
Introduction
In 1991, commercial lithium-ion batteries attracted the attention of researchers [1]. However, the limited lithium resources increased their cost. To deal with this problem, sodium-ion batteries became a research focus owing to abundant sodium resources and high electrode potential, and people turned their attention to sodium-ion batteries, which appeared almost simultaneously [2, 3]. Among the sodium resources, the transition metal oxides, NaXMO2 [4] with a layered structure and phosphate materials (NaFePO4 [5, 6] and Na3M2(PO4)3 [7, 8]) with a stable structure have become current hotspot materials. This is largely because layered metal oxides generally have higher capacity and larger interlayer spacing (e.g., NaCoO2, NaMnO2, and NaFeO2), and phosphate materials with a sodium super ionic conductor (NASICON) structure have high voltage and excellent structural thermal stability (e.g., NaFePO4 and Na3V2(PO4)3).
Because Na3V2(PO4)3 materials not only have ideal capacity, but also have high energy density and very stable NASICON structure [9], they are widely used in people’s production and life. At the same time, they have caused researcher’s attention too. In 1978, Delmas was the first to prepare a diamond-shaped Na3V2(PO4)3 using a high-temperature solid-phase method. Since then, more and more experts began to study Na3V2(PO4)3 [10,11,12,13] from various perspectives. They pointed out that Na3V2(PO4)3 not only played a very significant role in the production of sodium ion batteries, but also could be widely used in the production of hybrid-ion batteries. Some Na3V2(PO4)3 materials have also been successfully applied to hybrid-ion batteries. In 2013, Du, a research scholar, conducted an in-depth research and reflection on Na3V2(PO4)3, and proposed for the first time that it could be used as a cathode material in the manufacture of hybrid-ion batteries [14]. Subsequently, in 2015, Mai et al. reported that nanoflake-assembled Na3V2(PO4)3 could be prepared using a precipitation method, with remarkable rate performance [15]. In 2019, Zhang et al. comprehensively reviewed the main factors affecting sodium storage capabilities and proposed corresponding strategies to meliorate their sodium storage properties [16]. In addition, compounding and carbon coating with suitable carbon species is an efficacious way to ameliorate the energy storage capabilities of species based on the published articles [16,17,18,19].
Herein, we synthesized polyacrylonitrile-modified Na3V2(PO4)3, in which polyacrylonitrile is used as a carbon source to ameliorate the lithium storage performance of Na3V2(PO4)3 when lithium sheets were used as the negative electrodes and LiPF6/EC DMC EMC was used as electrolyte. At the same time, the effects of different calcination conditions and different polyacrylonitrile contents on the lithium storage performance of Na3V2(PO4)3 materials were also investigated.
Experimental method
Material synthesis
The specific synthesis process is to first put 2 mmol of V2O5 and 6.07 mmol of H2C2O4 • 2H2O into 20 ml of deionized water. Then set the temperature to 70 °C and stir them with magnetic stirring for an hour until V2O5 dissolves completely, resulting in a blue solution. Thereafter, 6 mmol of NaH2PO4 were put to the blue solution and stirred for 5 min. After that, 50 ml of n-propanol was quickly put to the above solution and stirred for 10–20 min. After that, the polyacrylonitrile with different mass ratios were dissolved into the same concentration and the same milliliter of dimethylformamide (DMF) solution, respectively, and the obtained solution was put to the previously obtained solution again, and stirred for 20 min. The final step is to set the temperature of the blast furnace to 70 °C, put the solution into it for drying process, and finally get the precursor of Na3V2(PO4)3/C. The temperature of the tube furnace (argon atmosphere) is set to 400 °C, and the precursors obtained are put into it for pre-decomposition. Four hours later, the calcination process was carried out at 700, 750, and 800 °C, respectively. After 8 h, Na3V2(PO4)3/C materials were obtained. The reactions involved in the experiment are as follows:
Structural characterization
The structure and morphology were characterized using an X-ray powder diffractometer (XRD, a Bruker D2 diffractometer with Cu Ka (λ = 1.5408 Å)) and a transmission electron microscope (TEM, JEN-2010FEE), respectively.
Electrochemical measurement
According to the weight ratio of 80:10:10, the composite material was blended with acetylene black, polyvinylidene fluoride (PVDF) and an appropriate amount of N-methylpyrrolidone (NMP) on aluminum foil to obtain Na3V2(PO4)3/C electrode plate. The vacuum-drying chamber is set to 110 °C and the obtained electrode sheets are put into it for drying. After 20 h, the obtained electrode sheets were cut into 10 mm electrode pads using a punch. The pads were fabricated into a button-type battery. The electrochemical performance was measured after the battery was kept standing for 24 h. Lithium was used as the reverse electrode; Celgard2400 as the diaphragm material; and 1 mol/L LiPF6/EC DMC EMC as the electrolyte (volume ratio 1:1:1). The battery pack is placed in the glove box (MB10), and then charged and discharged by the terrestrial system (CT2001A), i.e., the voltage ranges from 2.5 to 4.5 V, at constant current. On the electrochemical workstation (CHI 650D), the AC impedance is measured from 0.01 to 105 Hz, and the cyclic voltammetry is tested from 2.5 to 4.5 V with the scanning rate of 0.1 mV s−1.
Results and discussion
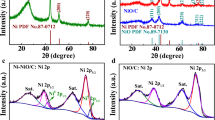
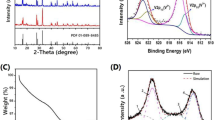
Figure 1 displays the XRD patterns of the Na3V2(PO4)3/C samples prepared under different conditions. Among them, Fig. 1a shows an XRD pattern of a sample containing 10 wt% of polyacrylonitrile calcined at different temperatures for 8 h. It is noted that Fig. 1b demonstrates a graph of samples containing different amounts of polyacrylonitrile but synthesized under the same calcination temperature and time conditions. It can be seen from Fig. 1 that the peak of the sample is consistent with the diffraction peak corresponding to the standard card (PDF#53-0018) and is in agreement with the results of previous studies. This alludes that the synthesized polyacrylonitrile-containing material is a pure phase Na3V2(PO4)3/C material of the R-3c space group. Further, in contradistinction to the crystal structure of the species containing different amounts of polyacrylonitrile, the results insinuate that as the amount of polyacrylonitrile increases; the phase composition and peak position as-prepared species are the same as the Na3V2(PO4)3 without polyacrylonitrile, and there are no conspicuous difference. This is because polyacrylonitrile is used as a carbon source in the synthesis process, which can generate an amorphous carbon layer during calcination at high temperature. In addition, Fig. 1a shows that as the calcination temperature increases, the peak intensity of the material containing 10 wt% of polyacrylonitrile gradually increased, and the peak shape is gradually sharpened indicating that the higher the temperature, the better the crystallinity of the material is. However, an excessive temperature will cause the material to agglomerate, which is not conducive to the diffusions of Li+ and Na+ during the reaction.
Characterization of Na3V2(PO4)3/C species by XPS can illustrate the elemental composition and valence state. The XPS test results of Na3V2(PO4)3/C species containing 10 wt% polyacrylonitrile contents are demonstrated in Fig. 2. Among them, Fig. 2a and b are the XPS full spectrum and partial map within 512–528 eV bond energy range of Na3V2(PO4)3/C species, respectively. In the full spectrum, when the bond energies are 1071.38, 531.08, 516.38, 284.78, and 133.28 eV, corresponding to Na1s, O1s, V2p, C1s, and P2p orbital bond energy of Na3V2(PO4)3/C species, respectively. In addition, the partial diagram manifested that when the bond energies are 524.28 eV and 516.78 eV, they are compatible with the bond energies of V2p1/2 and V2p3/2, correspondingly, which is consistent with the bond energy of trivalent vanadium. Thus, by analyzing the results expounded by the above XPS clarify that the Na3V2(PO4)3/C species we synthesized are pure phase, and V exists in the form of V3+ in the species.
The micro-characterization of the sample was obtained, which is the TEM picture of Na3V2(PO4)3/C material. In particular, in Fig. 3a, it can be clearly concluded that the material obtained by calcination at 750 °C for 8 h was a particle having a uniform size distribution, and no significant agglomeration occurred between the particles. Obvious lattice fringes can be seen from Fig. 3b, and when the lattice spacing is 0.37 nm, it corresponds to the (113) crystal plane of Na3V2(PO4)3. Moreover, an uneven carbon film with a thickness of 2.9 nm is coated on the surface of the Na3V2(PO4)3C material, as shown in Fig. 3b, owing to the carbonization of polyacrylonitrile. Based on an elemental analysis, the carbon content of the synthesized material under this condition was found to be 6.65%, further proving that the carbon was successfully coated on the material.
Figure 4a shows the first cycle charge/discharge capacity of the Na3V2(PO4)3/C materials containing different polyacrylonitrile contents under the same calcination conditions, wherein a, b, c, d, and e correspond to polyacrylonitrile content of 0, 5, 10, 15, and 20%. From the above figure, we can conclude that the initial discharge capacities of the samples containing 5, 10, and 15 wt% of polyacrylonitrile are 124.6, 130.9, and 123.6 mAh g−1, respectively, which are superior to the materials containing no polyacrylonitrile (101.5 mAh g−1). This is because the added polyacrylonitrile forms a hierarchical porous structure after carbonization [17]. Further, as the amount of polyacrylonitrile increases, lithium storage performance becomes more excellent. However, when the amount added exceeds 10 wt%, the specific discharge capacity in the first cycle has a decreasing tendency. In particular, the initial discharge capacity of the material containing 20 wt% of polyacrylonitrile is 98.7 mAh g−1, which is slightly inferior to that of the sample with no polyacrylonitrile. The possible reason is that the carbon content increased considerably, thereby hindering the reaction of the active material and the electrolyte. Hence, the optimum polyacrylonitrile additive amount is 10 wt%. Figure 4b shows that the first cycle discharge specific capacity of the Na3V2(PO4)3/C sample containing 10 wt% polyacrylonitrile calcined at different calcination temperatures for 8 h, with letters a, b, and c corresponding to 700, 750, and 800 °C, respectively. We can find out that as the calcination temperature increases, the electrochemical performance of the material first increases and then decreases, which is due to the further increase in the calcination temperature; the material appears to be agglomerated, and the particle size increases. Meanwhile, as shown in Fig. 4c and d, it can be concluded that the Na3V2(PO4)3/C annealed at 750 °C (10 wt% of polyacrylonitrile) material has better lithium storage properties with initial discharge specific capacities of 122.2, 111.4, and 100.2 mAh g−1 at different current densities of 1, 2, and 5 C, respectively; the corresponding coulomb efficiencies are 99.5, 99.9, and 82.6%. Even after 500 cycles, the discharge specific capacity remains high, which can achieve 113.3, 99.5, and 90.1 mAh g−1; the capacity retention rates are 92.7, 89.3, and 89.9%, respectively. The discharge specific capacity of the material exceeds its theoretical capacity (118 mAh g−1), mainly owing to the different mechanism that the Na3V2(PO4)3/C serves as the hybrid-ion battery [15, 20].
a The initial charge/discharge capacity of Na3V2(PO4)3/C sample with different polyacrylonitrile contents(a: 0%, b: 5%, c: 10%, d: 15%, e: 20%); b the initial charge/discharge capacity of Na3V2(PO4)3/C sample at different calcination temperatures(a: 700 °C, b: 750 °C, c: 800 °C); c the initial charge/discharge capacity of Na3V2(PO4)3/C sample at different rates (a: 5 C, b: 2 C, c: 1 C); d cycle life curves of Na3V2(PO4)3/C sample (a: 1 C, b: 2 C, c: 5 C)
It can be markedly seen from Fig. 5a that the third anterior CV cyclic curves of Na3V2(PO4)3 electrode can be obtained at a scanning speed of 0.1 mVs−1. Redox couples can be observed at a potential of approximately 3.87/3.65 V, which was applied to the V3+/V4+ reaction [15]. The first three cycles of the curves are in good agreement with each other, indicating that the material has good reversibility and stability during the electrochemical reaction, which corresponds to the charge/discharge voltage platform. The electrochemical impedance spectroscopy (EIS) of the Na3V2(PO4)3/C annealed at 750 °C (10 wt% of polyacrylonitrile) sample was then examined to explore the embedding and delaminating processes in the active material of the hybrid-ion battery. As shown in Fig. 5b, the Nyquist diagram of the material can be divided into two parts [21]. There are two kinds, the charge-transfer impedance in the high-frequency region of the semicircle and the Warburg impedance in the low-frequency region of the diagonal region. According to the experimental results, Zview software is used to simulate the material’s AC impedance equivalent circuit. Charge transfer impedance and Warburg impedance are 701.7 and 17.66 Ω, respectively.
Conclusions
Polyacrylonitrile-modified Na3V2(PO4)3 composites prepared using a precipitation method with further calcination in an Argon atmosphere were found to exhibit excellent charge/discharge and cycle performances. In particular, even at a rate of 5 C, the initial discharge capacities of the Na3V2(PO4)3/C composites with 10 wt% of polyacrylonitrile were found to be 100.2 and 90.1 mAh g−1, which are retained after 500 cycles. Hence, experimental results show that polyacrylonitrile can enhance the properties of Na3V2(PO4)3/C scientifically and effectively, and has broad application prospects.
References
Wakihara, M.: Recent developments in lithium ion batteries. Mater. Sci. Eng. R. 33(4), 109–134 (2001)
Xiang, X.D., Zhang, K., Chen, J.: Recent advances and prospects of cathode materials for sodium-ion batteries. Adv. Mater. 27(36), 5343–5364 (2015)
Palomares, V., Serras, P., Villaluenga, I., et al.: Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 5(3), 5884–5901 (2012)
Han, M.H., Gonzalo, E., Singh, G., et al.: A comprehensive review of sodium layered oxides: powerful cathodes for Na-ion batteries. Energy Environ. Sci. 8(1), 81–102 (2015)
Saurel, D., Kubiak, P., Palomares, V., et al.: Crystal chemistry of Na insertion/deinsertion in FePO4–NaFePO4. J. Mater. Chem. 22(34), 17421–17423 (2012)
Sun, A., Haynes, D., Narayanan, S.R., et al.: Synthesis, characterization, and electrochemical studies of chemically synthesized NaFePO4. Mater. Sci. Eng. B. 177(20), 1729–1733 (2012)
Kabbour, H., Coillot, D., Masquelier, C., et al.: α-Na3M2(PO4)3 (M= Ti, Fe): absolute cationic ordering in NASICON-type phases. J. Am. Chem. Soc. 133(31), 11900–11903 (2011)
Plashnitsa, L.S., Kobayashi, E., Noguchi, Y., et al.: Performance of NASICON symmetric cell with ionic liquid electrolyte. J. Electrochem. Soc. 157(4), A536–A543 (2010)
Saravanan, K., Mason, C.W., Rudola, A., et al.: The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 3(4), 444–450 (2013)
Gopalakrishnan, J., Rangan, K.K.: V2(PO4)3: a novel NASICON-type vanadium phosphate synthesized by oxidative deintercalation of sodium from Na3V2(PO4)3. Chem. Mater. 4(4), 745–747 (1992)
Cushing, B.L., Goodenough, J.B.: Li2NaV2(PO4)3: a 3.7 V lithium-insertion cathode with the rhombohedral NASICON structure. J. Solid State Chem. 162(2), 176–181 (2001)
Lim, S.Y., Kim, H., Shakoor, R.A., et al.: Electrochemical and thermal properties of NASICON structured Na3V2(PO4)3 as a sodium rechargeable battery cathode: a combined experimental and theoretical study. J. Electrochem. Soc. 159(9), A1393–A1397 (2012)
Rousse, G., David, R., Courty, M., et al.: Discovery of a sodium-ordered form of Na3V2(PO4)3 below ambient temperature. Chem. Mater. 27(17), 5982–5987 (2015)
Du, K., Guo, H., Hu, G., et al.: Na3V2(PO4)3 as cathode material for hybrid lithium ion batteries. J. Power Sources. 223(1), 284–288 (2013)
An, Q.Y., Xiong, F.Y., Wei, Q.L., et al.: Nanoflake-assembled hierarchical Na3V2(PO4)3/C microflowers: superior Li storage performance and insertion/extraction mechanism. Adv. Energy Mater. 5(10), 1401963 (2015)
Yu, Y., Zhang, X.H., Rui, X.H., et al.: Na3V2(PO4)3: an advanced cathode for sodium-ion batteries. Nanoscale. 11, 2556–2576 (2019)
Shi, M., Kong, L.B., Liu, J.B., et al.: A novel carbon source coated on C-LiFePO4 as a cathode material for lithium-ion batteries. Ionics. 22(2), 185–192 (2016)
Zhang, Y., Wang, Y.B., Su, Z.: Novel preparation and electrochemical characteristics of Li3V2(PO4)3/C composite materials. J. Aust. Ceram. Soc. 53(2), 539–543 (2017)
Zhang, Y., Jia, D.Z., Tang, Y.K., et al.: In situ chelating synthesis of hierarchical LiNi1/3 Co1/3Mn1/3O2 polyhedron assemblies with ultralong cycle life for Li-Ion batteries. Small. 14(27), 1704354 (2018)
Song, W.X., Ji, X.B., Pan, C.C., et al.: A Na3V2(PO4)3 cathode material for use in hybrid lithium ion batteries. Phys. Chem. Chem. Phys. 15(34), 14357–14363 (2013)
Cui, Y.L., Wang, M.Z., Wang, J.L., et al.: Effect of temperature on the electronic/ionic transport properties of porous LiNi0.5Mn1.5O4 with high voltage for lithium ion batteries. Mater. Chem. Phys. 180, 46–52 (2016)
Funding
The study was supported by the key project of scientific research plan of colleges and universities in Xinjiang(XJEDU2018I015) and the project of Xinjiang tianchi cedar (2018XS03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, M., Su, Z. & Tian, H. Superior lithium storage performance of polyacrylonitrile-modified NASICON structure Na3V2(PO4)3 materials. J Aust Ceram Soc 56, 1073–1077 (2020). https://doi.org/10.1007/s41779-020-00451-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-020-00451-7