Abstract
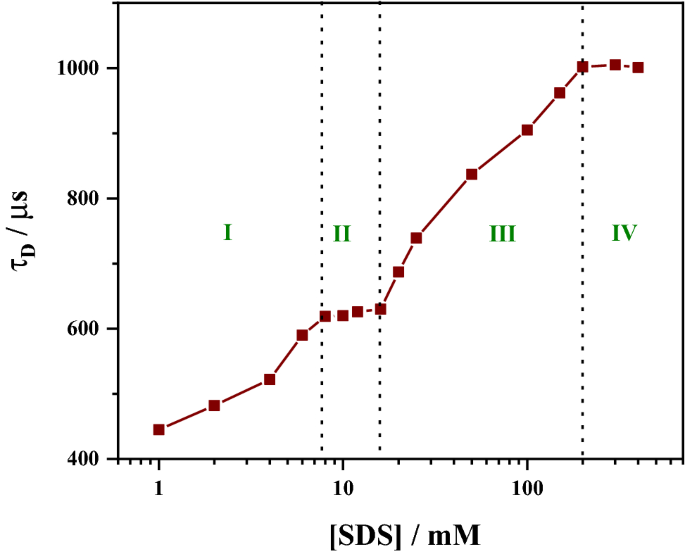
The denaturation of human serum albumin (HSA) upon interaction with the surfactant sodium dodecyl sulphate (SDS) was examined by measuring the diffusion time of fluorophore (RITC) tagged HSA under near single-molecule conditions using fluorescence correlation spectroscopy. The diffusion time shows four distinct regions as a function of SDS concentration, which corresponds to (I) opening of the tertiary structure, (II) non-specific SDS aggregation, (III) opening of the secondary structure, and (IV) aggregation of SDS around the secondary structure. Diffusion time increases from 383 µs for the free protein to 1002 µs for the SDS bound protein, which leads to an effective increase in the hydrodynamic radius by a factor of about 2.6.
Graphic abstract
Fluorescence correlation spectroscopy reveals a four-step interaction regime between SDS and HSA. The initial opening of the tertiary structure, followed by non-specific aggregation and opening up the secondary structure and finally leading to formation of protein bound micelles with an effective increase of hydrodynamic radius by a factor of 2.6.



Similar content being viewed by others
References
Otzen D 2011 Protein–surfactant interactions: a tale of many states Biochim. Biophys. Act. (BBA) 1814 562
Cooper A and Kennedy M W 2010 Biofoams and natural protein surfactants Biophys. Chem. 151 96
Vasilescu M and Angelescu D 1999 Interactions of globular proteins with surfactants studied with fluorescence probe methods Langmuir 15 2635
Gelamo E L and Tabak M 2000 Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants Spectrochim. Acta Part A 56 2255
Vlasova I M and Saletsky A M 2011 Polarized fluorescence in the study of rotational diffusion of human albumin during denaturation under the action of SDS Moscow Univ. Phys. Bull. 1 58
Vlasova I M and Saletsky A M 2009 Fluorescence of tryptophan in the denaturation of human serum albumin under the action of sodium dodecyl sulfate Russ. J. Phys. Chem. B 3 976
Anand U, Jash C and Mukherjee S 2010 Spectroscopic probing of the microenvironment in a protein-surfactant assembly J. Phys. Chem. B 114 15839
Hazra P, Chakrabarty D, Chakraborty A and Sarkar N 2004 Probing protein–surfactant interaction by steady state and time-resolved fluorescence spectroscopy Biochem. Biophys. Res. Commun. 314 543
Turro N J, Lei X G, Ananthapadmanabhan K P and Aronson M 1995 Spectroscopic probe analysis of protein-surfactant interactions: the BSA/SDS system Langmuir 11 2525
Shweitzer B, Zanette D and Itri R 2004 Bovine serum albumin (BSA) plays a role in the size of SDS micelle-like aggregates at the saturation binding: the ionic strength effect J. Coll. Inter. Sci. 277 285
Santos S F, Zanette D, Fischer H and Itri R 2003 A systematic study of bovine serum albumin (BSA) and sodium dodecyl sulfate (SDS) interactions by surface tension and small angle X-ray scattering J. Colloid Interface Sci. 262 400
Moriyama Y, Ohta D, Hachiya K, Mitsui Y and Takeda K 1996 Fluorescence behavior of tryptophan residues of bovine and human serum albumins in ionic surfactant solutions: a comparative study of the two and one tryptophan(s) of bovine and human albumins J. Prot. Chem. 15 265
Moriyama Y and Takeda K 1999 Re-formation of the helical structure of human serum albumin by the addition of small amounts of sodium dodecyl sulfate after the disruption of the structure by urea. A comparison with bovine serum albumin Langmuir 15 2003
Moriyama Y, Kanasaka and Takeda K 2003 Protective effect of small amounts of sodium dodecyl sulfate on the helical structure of bovine serum albumin in thermal denaturation J. Colloid Interface Sci. 257 41
Chatterjee S and Mukherjee T K 2016 Insights into the morphology of human serum albumin and sodium dodecyl sulfate complex: a spectroscopic and microscopic approach J. Colloid Interface Sci. 478 29
Mukherjee T K, Lahiri P and Datta A 2007 2-(2′-Pyridyl)benzimidazole as a fluorescent probe for monitoring protein–surfactant interaction Chem. Phys. Lett. 438 218
Reynolds J A and Tanford 1970 Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes Proc. Natl. Acad. Sci. U.S.A. 66 1002
Allen G 1974 The binding of sodium dodecyl sulphate to bovine serum albumin at high binding ratios Biochemistry J. 137 575
Laurie O and Oakes J 1975 Protein-surfactant interactions. Spin label study of interactions between bovine serum albumin (BSA) and sodium dodecyl sulphate (SDS) J. Chem. Soc. Faraday Trans. 1 71 1324
Oakes J 1974 Protein-surfactant interactions. Nuclear magnetic resonance and binding isotherm studies of interactions between bovine serum albumin and sodium dodecyl sulphate J. Chem. Soc. Faraday Trans. 1 70 2200
Shinagawa S, Sato M, Kameyama K and Takagi T 1994 Effect of salt concentration on the structure of protein-sodium dodecyl sulfate complexes revealed by small-angle X-ray scattering Langmuir 10 1690
Santra M K, Banerjee A, Rahaman O and Panda 2005 Unfolding pathways of human serum albumin: evidence for sequential unfolding and folding of its three domains Int. J. Biol. Macromol. 37 200
He X M and Carter D C 1992 Atomic structure and chemistry of human serum albumin Nature 358 209
Dugaiczyk A, Law S W and Dennison O E 1982 Nucleotide sequence and the encoded amino acids of human serum albumin mRNA Proc. Natl. Acad. Sci. U.S.A. 79 71
Green A M and Abelt C J 2015 Dual-sensor fluorescent probes of surfactant-induced unfolding of human serum albumin J. Phys. Chem. B 119 3912
Yadav R, Sengupta B and Sen P 2014 Conformational fluctuation dynamics of domain I of human serum albumin in the course of chemically and thermally induced unfolding using fluorescence correlation spectroscopy J. Phys. Chem. B 118 5428
Magde D, Elson E and Webb W W 1972 Thermodynamic fluctuations in a reacting system-measurement by fluorescence correlation spectroscopy Phys. Rev. Lett. 29 705
Elson E L and Magde D 1974 Fluorescence correlation spectroscopy. I. Conceptual basis and theory Biopolymers 13 1
Widengren J, Dapprich J and Rigler R 1997 Fast interactions between Rh6G and dGTP in water studied by fluorescence correlation spectroscopy Chem. Phys. 216 417
Haustein E and Schwille P 2007 Fluorescence correlation spectroscopy: novel variations of an established technique Annu. Rev. Biophys. Biomol. Struct. 36 151
Wöll D 2014 Fluorescence correlation spectroscopy in polymer science RSC Adv. 4 2447
Rathgeber S, Beauvisage H J, Chevreau H, Willenbacher N and Oelschlaeger C 2009 Microrheology with fluorescence correlation spectroscopy Langmuir 25 6368
Kim S 2003 Intracellular applications of fluorescence correlation spectroscopy: prospects for neuroscience Curr. Opin. Neurobiol. 13 583
Wachsmuth M, Waldeck W and Langowski J 2000 Counting nucleosomes in living cells with a combination of fluorescence correlation spectroscopy and confocal imaging J. Mol. Biol. 298 677
Sengupta P, Balaji J and Maiti S 2002 Measuring diffusion in cell membranes by fluorescence correlation spectroscopy Methods 27 374
Sengupta P, Garai K, Balaji J, Periyasami N and Maiti S 2003 Measuring size distribution in highly heterogeneous systems with fluorescence correlation spectroscopy Biophys. J. 84 1977
Al-soufi W, Novo M, Felekyan S and Seidel C A M 2005 Fluorescence correlation spectroscopy, a tool to investigate supramolecular dynamics: inclusion complexes of pyronines with cyclodextrin J. Am. Chem. Soc. 109 8775
Al-Soufi W, Reija B, Felekyan S, Seidel C M and Novo M 2008 Dynamics of supramolecular association monitored by fluorescence correlation spectroscopy Chem. Phys. Chem. 9 1819
Sherman E, Itkin A, Kuttner Y Y, Rhoades E, Amir D, Haas E and Haran G 2008 Using fluorescence correlation spectroscopy to study conformational changes in denatured proteins Biophys. J. 94 4819
Sen Mojumdar S, Chowdhury R, Chattoraj S and Bhattacharyya K 2012 J. Phys. Chem. B 116 12189
Sasmal D K, Mondal T, Sen Mojumdar S, Choudhury A, Banerjee R and Bhattacharyya K 2011 An FCS study of unfolding and refolding of CPM-labeled human serum albumin: role of ionic liquid J. Phys. Chem. B 115 13075
Ghosh S, Adhikari A, Sen Mojumdar S and Bhattacharyya K 2010 A fluorescence correlation spectroscopy study of the diffusion of an organic dye in the gel phase and fluid phase of a single lipid vesicle J. Phys. Chem. B 114 5736
Rigler R and Elson E S 2000 Fluorescence Correlation Spectroscopy: Theory and Applications Springer Series in Chemical Physics vol. 65 (Berlin: Springer Verlag)
Magde D, Elson E L and Webb W W 1974 Fluorescence correlation spectroscopy. I. Conceptual basis and theory. Fluorescence correlation spectroscopy conceptual basis and theory. II Biopolymers 13 29
Jachimska B, Wasilewska M and Adamczyk Z 2008 Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements Langmuir 24 6866
Leggio C, Galantini L, Konarev P V and Pavel N V 2009 Urea-induced denaturation process on defatted human serum albumin and in the presence of palmitic acid J. Phys. Chem. B 113 12590
Anand U, Ray S, Ghosh S, Banerjee R and Mukherjee S 2015 Structural aspects of a protein–surfactant assembly: native and reduced states of human serum albumin Protein J. 34 147
Zhang X, Poniewierski A, Hou S, Sozanski K, Wisniewska A, Wieczorek S A, Kalwarczyk T, Sun L and Hołyst R 2015 Tracking structural transitions of bovine serum albumin in surfactant solutions by fluorescence correlation spectroscopy and fluorescence lifetime analysis Soft Matter 11 2512
Acknowledgements
Authors wish to thank Prof. Sudipta Maiti for his help in setting up the FCS instrument as part of the first FCS workshop held at TIFR in the year 2009. The FCS instrument was procured as part of the Department of Information Technology Govt. of India sponsored research project entitled Construction and multi-site commissioning of multiple fluorescence correlation spectrometers [Project No. 12(4)/2007-PDD]. VS gratefully acknowledges CSIR for fellowship.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samant, V., Dey, A. & Naresh Patwari, G. Probing the interaction between human serum albumin and the sodium dodecyl sulphate with fluorescence correlation spectroscopy. J Chem Sci 132, 109 (2020). https://doi.org/10.1007/s12039-020-01816-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01816-y




