Abstract
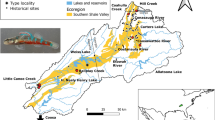
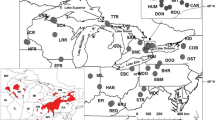
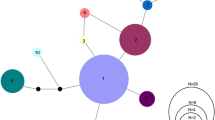
The southeastern United States harbors one of the most diverse temperate freshwater fish faunas of the world. Unfortunately, due to improper land use practices and habitat degradation, many of the species in this region are imperiled and may become extinct without appropriate conservation efforts. This study examined the population dynamics of an endangered endemic darter of southwest Kentucky, the Relict Darter (Etheostoma chienense) and its sister taxon, the undescribed Clarks Darter (Etheostoma cf. oophylax). Mitochondrial sequence data coupled with SNP data were used to infer population structure, gene flow, genetic variation, and effective population sizes of both species. The results from this study, based on 160 individuals from nine localities, indicate that the endangered Relict Darter possesses limited genetic variation based on mitochondrial DNA haplotypes (N = 4). In addition, SNP data (6.8 k markers) further indicates limited genetic structure (K = 1), as well as a low effective population size (143–918), suggesting that the Relict Darter can be managed as a single, panmictic conservation unit. It is suggested that conservation efforts be taken to protect remaining habitats, augment the system with artificial spawning substrates, and, as a last resort (if needed), supplement the natural population with captive reared individuals. Ethesotoma cf. oophylax showed some genetic variation among distant sites, but more samples are needed throughout the range in order to fully understand the population dynamics of this species.





Similar content being viewed by others
References
Belmar-Lucero S, Wood JL, Scott S, Harbicht AB, Hutchings JA, Fraser DJ (2012) Concurrent habitat and life history influences on effective/census population size ratios in stream-dwelling trout. Ecol Evol 2:562–573
Berthier P, Beaumont MA, Cornuet JM, Luikart G, Anderson EC, Williamson EG, Wright S (2002) Likelihood-based estimation of the effective population size using temporal changes in allele frequencies: a genealogical approach. Genetics 160:741–751
Camak DT, Piller KR (2018) Going with the flow: testing the role of habitat isolation among three ecologically divergent darter species. Copeia 106:375–387
Chhatre VE, Emerson KJ (2017) StrAuto: automation and parallelization of STRUCTURE analysis. BMC Bioinform 18:192
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Douglas M, Keck BP, Ruble C, Petty M, Shute JR, Rakes P, Hulsey CD (2013) Pelagic larval duration predicts extinction risk in a freshwater fish clade. Biol Lett 9:20130672
Earl DA (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Eaton DA, Overcast I (2020) ipyrad: interactive assembly and analysis of RADseq datasets. Bioinformatics 36:2592–2594
Eliades NGH, Gailing O, Leinemann L, Fady B, Finkeldey R (2011) High genetic diversity and significant population structure in Cedrus brevifolia Henry, a narrow endemic Mediterranean tree from Cyprus. Plant Syst Evol 294:185–198
Evans JD, Page LM (2003) Distribution and relative size of the swim bladder in Percina, with comparisons to Etheostoma, Crystallaria, and Ammocrypta (Teleostei: Percidae). Environ Biol Fishes 66:61–65
Faulks LK, Gilligan DM, Beheregaray LB (2010) Islands of water in a sea of dryland: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the Golden Perch (Macquaria ambigua). Mol Ecol 19:4723–4737
Floyd M (2018) Five year review: Relict Darter (Etheostoma chienense). Kentucky Ecological Services Field, Frankfurt Kentucky
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Frankham R (2010) Challenges and opportunities of genetic approaches to biological conservation. Biol Conserv 143:1919–1927
Frankham R, Bradshaw CJA, Barry BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol Conserv 170:56–63
Frankham R (2015) Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol 24:2610–2618
Franklin R (1980) Evolutionary change in small populations. In: Soule ME, Wilcox BA (eds) Conservation biology: an evolutionary-ecological perspective. Sinauer Associates, Sunderland, pp 135–140
Franklin IR, Allendorf FW, Jamieson IG (2014) The 50/500 rule is still valid-reply to Frankham et al. Biol Conserv 176:284–285
Gido KB, Whitney JE, Perkin JS, Turner TF (2016) Fragmentation, connectivity and fish species persistence in freshwater ecosystems. In: Krkosek M, Olden JD, Closs GP (eds) Conservation of Freshwater Fishes. Cambridge University Press, Cambridge, pp 292–323
Goudet J (2005) Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 5(1):184–186
Harrington RC, Benavides E, Near TJ (2013) Phylogenetic inference of nuptial trait evolution in the context of asymmetrical introgression in North American darters (Teleostei). Evolution 67:388–402
Harrington RC, Near TJ (2015) Phylogenetic relationships of Goneaperca and the evolution of parental care in darters (Teleostei:Percidae). Mol Phylogenet Evol 84:158–165
Huey JA, Balcombe SR, Real KM, Sternberg D, Hughes JM (2017) Genetic structure and effective population size of the most northern population of the Australian River Blackfish, Gadopsis marmoratus (Richardson 1848): implications for long-term population viability. Freshw Sci 36:113–123
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Jamieson IG, Allendorf FW (2012) How does the 50/500 rule apply to MVPs? Trends Ecol Evol 27:578–584
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27:3070–3071
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Liu JX, Gao TX, Zhuang ZM, Jin XS, Yokogawa K, Zhang YP (2006) Late Pleistocene divergence and subsequent population expansion of two closely related fish species, Japanese anchovy (Engraulis japonicus) and Australian Anchovy (Engraulis australis). Mol Phylogenet Evol 40:712–723
Mee JA, Bernatchez L, Reist JD, Rogers SM, Taylor EB (2015) Identifying designatable units for intraspecific conservation prioritization: a hierarchical approach applied to the Lake Whitefish species complex (Coregonus spp.). Evol Appl 8:423–441
Near TJ, Bossu CM, Bradburd GS, Carlson RL, Harrington RC, Hollingsworth PR Jr, Keck BP, Etnier DA (2011) Phylogeny and temporal diversification of darters (Percidae: Etheostomatinae). Syst Biol 60:565–595
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Page LM (1983) Handbook of Darters. TFH Publications, Neptune City
Page LM, Ceas PA, Swofford DL, Buth DG (1992) Evolutionary relationships within the Etheostoma squamiceps complex (Percidae; subgenus Catonotus) with descriptions of five new species. Copeia 1992:615–646
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20(2):289–290
Perkin JS, Gido KB (2012) Fragmentation alters stream fish community structure in dendritic ecological networks. Ecol Appl 22:2176–2187
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7:e37135
Pilger TJ, Gido KB, Propst DL, Whitney JE, Turner TF (2017) River network architecture, genetic effective size and distributional patterns predict differences in genetic structure across species in a dryland stream fish community. Mol Ecol 26:2687–2697
Piller KR, Burr BM (1998) Distribution and population estimates of the federally endangered Relict Darter, Etheostoma chienense, Bayou du Chien, Kentucky. J Kentucky Acad Sci 59:64–75
Piller KR, Burr BM (1999) Reproductive biology and spawning habitat supplementation of the relict darter, Etheostoma chienense, a federally endangered species. Environ Biol Fishes 55:145–155
Piller KR, Wilson CC, Lee CE, Lyons J (2005) Conservation genetics of inland Lake Trout in the upper Mississippi River basin: stocked or native ancestry? Trans Am Fish Soc 134:789–802
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rafiei V, Banihashemi J-Díaz RM, Navas-Cortés JA, Landa BB, Jiménez-Gasco MM, Turgeon BG, Milgroom MG (2018) Comparison of genotyping by sequencing and microsatellite markers for unravelling population structure in the clonal fungus Verticillium dahliae. Plant Pathol 67:76–86
Rosenberg NA (2004) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Rosenfeld JS (2014) 50/500 or 100/1000? Reconciling short- and long-term recovery targets and MVPs. Biol Conserv 176:287–288
Russello MA, Waterhouse MD, Etter PD, Johnson EA (2015) From promise to practice: pairing non-invasive sampling with genomics in conservation. PeerJ 3:1106
Schmidt TR, Gold JR (1993) Complete sequence of the mitochondrial cytochrome b gene in the cherryfin shiner, Lythrurus roseipinnis (Teleostei: Cyprinidae). Copeia 1993 (3):880
Scribner KT, Lowe WH, Landguth E, Luikart G, Infante DM, Whelan GE, Muhlfeld CC (2016) Applications of genetic data to improve management and conservation of river fishes and their habitats. Fisheries 41:174–188
Shaffer ML (1981) Minimum population sizes for species conservation. Bioscience 31:131–134
Soule ME (1980) Thresholds for survival: maintaining fitness and evolutionary potential. In: Soule ME, Wilcox BA (eds) Conservation biology: an evolutionary-ecological perspective. Sinauer Associates, Sunderland, pp 151–169
Thomaz AT, Christie MR, Knowles LL (2016) The architecture of river networks can drive the evolutionary dynamics of aquatic populations. Evolution 70:731–739
Turner TF, Wares JP, Gold JR (2002) Genetic effective size is three orders of magnitude smaller than adult census size in an abundant, estuarine-dependent marine fish (Sciaenops ocellatus). Genetics 162:1329–1339
Von der Heyden S, Lipinski MR, Matthee CA (2007) Mitochondrial DNA analyses of the Cape Hakes reveal an expanding, panmictic population for Merluccius capensis and population structuring for mature fish in Merluccius paradoxus. Mol Phylogenet Evol 42:517–527
Vrijenhoek RC (2005) Conservation genetics of freshwater fish. J Fish Biol 53(Suppl A):394–412
Wahlund S (1928) Zusammensetzung von Populationen und Korrelationserscheinungen vom Standpunkt der Vererbungslehre aus betrachtet. Hereditas 11:65–106
Waples RS, Do C (2010) Linkage disequilibrium estimates of contemporary Ne using highly variable genetic markers: a largely untapped resource for applied conservation and evolution. Evol Appl 3:244–262
Waples RS (2014) Testing for Hardy-Weinberg proportions: have we lost the plot? J Hered 106:1–19
Warren ML, Burr BM, Taylor CA (1994) The Relict Darter, Etheostoma chienense (Percidae): status review of a Kentucky endemic. Trans Kentucky Acad Sci 55:20–27
Warren ML Jr, Burr BM, Walsh SJ, Bart HL Jr, Cashner RC, Etnier DA, Freeman BJ, Kuhajda BR, Mayden RL, Robison HW, Ross ST (2000) Diversity, distribution, and conservation status of the native freshwater fishes of the southern United States. Fisheries 25:7–31
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38 (6):1358
Westemeier RL, Brawn JD, Simpson SA, Esker TL, Jansen RW, Walk JW, Kershner EL, Bouzat JL, Paige KN (1998) Tracking the long-term decline and recovery of an isolated population. Science 282:1695–1698
Zhivotovsky LA (2015) Relationships between Wright’s FST and FIS statistics in a context of Wahlund effect. J Hered 106:306–309
Acknowledgements
We would like to thank Anna Gruszkiewicz, Aaron Krolow, and Arely Ramirez for assistance in the field. We would also like to thank Mike Floyd, Mike Compton, and Matt Thomas for providing tissue samples from several Relict Darter localities. Members of the Near Lab at Yale University, Ava Ghezelayagh, Daemin Kim, Dan MacGuigan, Tom Near, & Rich Harrington provided ddRAD library prep training and assistance with the ipyrad assembly of raw Illumina data. I would also like to thank David Camak for his guidance with analysis of this data. Funding was provided through a grant to KRP from the Kentucky Department of Fish and Wildlife Resources via the Cooperative Endangered Species Conservation Fund (Section 6 of the Endangered Species Act). Additional logistical support was provided by the U.S. Fish and Wildlife Service, Kentucky Ecological Services Field Office. Portions of this research were conducted with high performance computational resources provided by the Louisiana Optical Network Infrastructure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kattawar, J., Piller, K.R. Comparative population genetics of the federally endangered Relict Darter, and its sister taxon the Clarks Darter (Teleostei: Percidae). Conserv Genet 21, 957–970 (2020). https://doi.org/10.1007/s10592-020-01300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-020-01300-7