Abstract
Experimental study on the phase equilibria between copper matte with silica-saturated iron silicate slags was conducted at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.5 atm. The high-temperature isothermal equilibration in silica crucibles under controlled flowing CO-CO2-SO2-Ar was followed by quenching in an ice–water mixture and direct phase composition analyses by an electron probe X-ray microanalyzer. The equilibrium compositions for matte and slag, as well as the distribution coefficients, were displayed as a function of matte grade. The data set obtained at \( P_{{{\text{SO}}_{ 2} }} \) = 0.5 atm and the previous study at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm by the authors enabled an investigation on the impacts of \( P_{{{\text{SO}}_{ 2} }} \) as well as Al2O3 and CaO additions on phase equilibria in the multiphase copper matte smelting system. Thermodynamic calculations using MTDATA software were performed to compare the experimental results with modeling. The present results enrich the fundamental thermodynamic information for the matte/slag/tridymite/gas equilibria in the primary copper smelting process at high \( P_{{{\text{SO}}_{ 2} }} \).
Similar content being viewed by others
Introduction
Copper flash smelting has been the leading technology for primary copper sulfide smelting[1] because of its low energy consumption and environmental impact, reduced operational costs, and high on-line availability.[2,3] The \( P_{{{\text{SO}}_{ 2} }} \), generated by the slag and matte formation reactions, in the horizontal settler of the flash smelting furnace (FSF) is equal to the prevailing total pressure.[4,5] Since the late 1970s, the smelting capacity of the FSF has been increased by 100 to 200 pct since technical oxygen (> 95 pct O2) was adopted into copper smelting,[2,6] resulting in increased SO2 content of the off-gas to > 50 vol pct SO2. Therefore, accurate phase equilibria information of the copper matte and slag under a high \( P_{{{\text{SO}}_{ 2} }} \) is essential for the copper smelting process from the standpoint of resource efficiency as well as stable and optimized operation.
The main components of the copper matte-slag equilibrium are described by the Cu-Fe-S-O-SiO2 system. However, industrial iron silicate-based copper smelting slags contain alumina (Al2O3), lime (CaO), and magnesia (MgO),[7,8] introduced into the smelting by copper ores, fluxes, and secondary raw materials (such as Waste Electrical and Electronic Equipment, WEEE). Hence, it is essential to extend the studies on matte-slag equilibria from the basic subsystem to the more complicated Cu-Fe-S-O-SiO2-Al2O3-MgO-CaO system under higher SO2 partial pressures with the aim of providing comprehensive experimental data for copper smelting and industrial practices.
A careful literature review indicated that phase equilibria of copper matte and slag over the past few decades have been studied mainly under the SO2 partial pressure of 0.1 atm[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23] related to air blowing, while only limited data were reported for the equilibria under higher \( P_{{{\text{SO}}_{ 2} }} \).[18,19,20,21,22,23] Roghani et al.[19,20,21,22] investigated the phase equilibria of copper matte and different slags with the presence of trace elements at 1250 °C to 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) of 0.1, 0.5, and 1 atm. They reported that the concentrations of copper, sulfur, and iron in slags were not affected by the prevailing \( P_{{{\text{SO}}_{ 2} }} \), similar to the observations by Tavera et al.[18] and Takeda.[23] However, the relationship between the matte/slag compositions and the \( P_{{{\text{SO}}_{ 2} }} \) of the system was not fully characterized in those studies. In previous studies at higher \( P_{{{\text{SO}}_{ 2} }} \),[18,19,20,21,22,23] the chemical analysis technique was used for measuring the matte and slag compositions, which gave somewhat scattered results due to incomplete separation of matte and slag. In recent publications by Fallah-Mehrjardi et al.[24,25,26,27] for the matte/slag/tridymite equilibria of the Cu-Fe-S-O-SiO2[24,25,26] and Cu-Fe-S-O-SiO2-CaO[27] systems at \( P_{{{\text{SO}}_{ 2} }} \) = 0.25 atm and t = 1200 °C to 1250 °C, the experiments were conducted using a high-temperature equilibration and quenching technique in flowing CO-CO2-SO2-Ar gas. The equilibrated phase compositions were analyzed directly on the polished sections of the matte and slag phases by EPMA (electron probe X-ray microanalysis).
Abdeyazdan et al.[28,29] investigated the effects of MgO and Al2O3 on the matte-slag-tridymite equilibria at 1200 °C to 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.25 atm. Shishin et al.[30] equilibrated copper matte and FeOx-SiO2 slags at tridymite saturation under \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 to 0.6 atm and t = 1200 °C to 1300 °C. They also studied the impacts of Al2O3, MgO, and CaO on the phase equilibrium of matte and slag with tridymite saturation at 1200 °C and \( P_{{{\text{SO}}_{ 2} }} \) of 0.25 atm.[31] It was found that the dissolution of copper, sulfur, and “FeO” in slag decreased with increasing MgO, Al2O3, and CaO concentration in slags, whereas the matte composition was not significantly affected by the slag modifiers.[28,29,31]
Hidayat et al.[32,33] investigated the matte-slag-spinel equilibria in the Cu-Fe-O-S-SiO2 system at \( P_{{{\text{SO}}_{ 2} }} \) of 0.25 atm and t = 1200°C. Chen et al.[34] investigated the phase equilibria of copper matte and FeOx-SiO2 slag with spinel saturation at 1200 °C to 1250 °C, \( P_{{{\text{SO}}_{ 2} }} \) = 0.3 and 0.6 atm, and fixed matte grade of 72 wt pct Cu. Sun et al.[35] and Chen et al.[34] studied the effects of CaO on the matte-slag-spinel equilibria and liquidus contours of slags under the same conditions but at different temperatures at 1150 °C to 1250 °C. They reported that the liquidus temperature increased with increasing CaO at constant SiO2 concentration. In addition, the equilibrium SiO2 concentration was found to increase significantly with increasing \( P_{{{\text{SO}}_{ 2} }} \).
In our previous works,[11,12,13,14,15] matte-slag equilibria at silica saturation have been studied at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm and t = 1250 °C to 1300°C with the presence of different trace elements. Therefore, the objective of the present study was to enrich the experimental thermodynamic data for equilibrium phase relations of copper matte and different silica-saturated FeOx-SiO2, FeOx-SiO2-Al2O3, and FeOx-SiO2-Al2O3-CaO slags at 1300 °C and high \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm. The use of silica crucibles ensured the silica saturation of the matte-slag system and provided the possibility to examine the system without extra impurities, such as MgO or Al2O3, introduced by the substrate. The new experimental data obtained in the present study can be used to update the multi-component and multi-phase databases of related thermodynamic software for copper smelting modeling.
Experimental
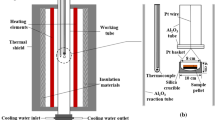
The experimental technique used in the present study involved high-temperature isothermal equilibration in controlled CO-CO2-Ar-SO2 atmospheres, quenching, and direct phase composition measurement of the phases using EPMA. The experiments were carried out in a vertical tube furnace (Lenton PTF 14/45/450) equipped with SiC heating elements and a recrystallized alumina work tube. The temperature of the three-zone furnace was regulated by three Eurotherm 3216 PID controllers. The temperature of samples in the furnace was measured by a calibrated S-type Pt/10 pctRh thermocouple (Johnson-Matthey Noble Metals, UK), located next to the silica crucible. Detailed information about the experimental methodology used has been described previously.[11,12,13,14,15]
The partial pressure of SO2 was held constant at 0.5 atm in all experiments. To create the designed O2 and S2 partial pressures in the gas mixture for different target matte grades, the gas flow rates were calculated by MTDATA[36] software using the SGTE pure substances database.[37] The calculated partial pressures and gas flow rates are listed in Table I. All gas flow rates were controlled by DFC26 digital mass flow controllers (Aalborg, USA).
The initial copper matte mixtures were synthesized from high-purity powders of Cu2S (99.5 wt pct) and FeS (99.9 wt pct). The silica-saturated iron silicate slags were prepared by mixing Fe2O3 (99.998 wt pct) and SiO2 (99.995 wt pct) from Alfa Aesar and Al2O3 (99.99 wt pct) and CaO (99.9 wt pct) from Sigma-Aldrich.[14,15] All gases used in this study were supplied by AGA-Linde (Finland) with purities > 99.97 vol pct. For each experiment, approximately 0.1 g copper matte mixture with an equal amount of slag mixture was pressed into a pellet before being placed into a silica crucible. The sample pellet was annealed under different gas atmospheres for 4 hour to get uniform matte and slag compositions, as suggested in our previous study.[14,15] It should be noted that all experiments were duplicated for increased reliability (4 × 2 × 3 = 24 in total). The equilibrated samples were quenched into ice–water mixture so that the phases presented at high temperature and their compositions were attained at room temperature. The quenched samples were cut into half, mounted in epoxy resin (EpoFix, Struers, Denmark), ground by SiC papers, and polished using a metallographic polishing cloth with diamond spray. The polished surfaces were carbon coated using a LEICA EM SCD050 sputtering device (Leica Microsystems, Austria) for electrical conductivity.
The micrographs of the polished sections and the preliminary phase compositions were examined using a scanning electron microscope, SEM (Tescan MIRA 3, Brno, Czech Republic) equipped with an UltraDry Silicon Drift Energy Dispersive X-ray Spectrometer, EDS (Thermo Fisher Scientific, Waltham, MA, USA). The elemental concentrations in matte and slag were analyzed with a Cameca SX100 EPMA (Cameca SAS, Genevilliers, France) fitted with five wavelength-dispersive spectrometers (WDS). The EPMA analyses were conducted at an accelerating voltage of 20 kV, beam current of 60 nA, and beam diameter of 100 µm for matte and 50 to 100 µm for slag. The employed standards and x-ray lines were quartz (Si Kα), almandine (Al Kα), metallic copper (Cu Kα), hematite (Fe Kα and O Kα), pentlandite (S Kα), and diopside (Ca Kα). The elemental detection limits of EPMA for matte and slag are presented in Table II. At least eight points were measured in both the matte and slag phase in each sample for statistical reliability. The raw data were processed by a PAP-ZAF on-line matrix correction program[38] before normalizing the results.
Results and Discussion
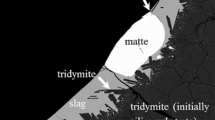
The experiments were carried out for a wide target matte grade range of 60 to 75 wt pct Cu at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm. Typical microstructures of the equilibrated phases obtained at \( P_{{{\text{O}}_{ 2} }} \) = 10−7.56 atm for target matte grade of 60 wt pct Cu are shown in Figure 1. The matte-slag equilibrium was found in all samples. A large fraction of recrystallized silica crystals, i.e., tridymite (> 99 wt pct SiO2), was precipitated in the slags, attributed to the silica saturation caused by the silica crucible. The tridymite preferred to form on the slag-crucible interfaces, but some discrete rod-like crystals also existed randomly in the slag. All phases were homogeneous after rapid quenching, except for the matte, where copper-rich veins (90 to 97 wt pct Cu) were found. The copper-rich veins were formed because of the insufficient quenching rate of sulfur-deficient matte. The measured average compositions of matte and liquid slag phases with standard deviations are listed in Table III.
Typical back-scattered electron micrographs of the matte-slag-silica system at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.5 atm; (a) 57.10 wt pct Cu in matte without Al2O3 and CaO in slag; (b) 61.1 wt pct Cu in matte with Al2O3-containing slag; (c) 64.4 wt pct Cu in matte with (Al2O3 + CaO)-containing slag
Matte Composition
The concentrations of copper, iron, sulfur, and oxygen in matte against oxygen partial pressure or matte grade are shown in Figure 2. The data for 0.1 atm \( P_{{{\text{SO}}_{ 2} }} \) are from our previous study.[15] As Figure 2(a) shows, the copper concentration in matte increased with increasing oxygen partial pressure, similar to the results at \( P_{{{\text{SO}}_{ 2} }} \) of 0.1 atm[13,15] and the observations by other researchers.[16,21,25,26,27,28,29,30] It was noted, in general, that the prevailing \( P_{{{\text{O}}_{ 2} }} \) increased with increasing \( P_{{{\text{SO}}_{ 2} }} \) at a given matte grade, as described in the sulfur-oxygen potential diagram by Yazawa.[17] A very similar relationship between the oxygen partial pressure and matte grade at different \( P_{{{\text{SO}}_{ 2} }} \) was also observed by Roghani et al.,[21] in which copper matte was equilibrated with FeOx-SiO2-MgO slag at \( P_{{{\text{SO}}_{ 2} }} \) of 0.1 to 1 atm. The \( P_{{{\text{O}}_{ 2} }} \) for different target matte grades in the present study was somewhat lower than the values reported by Roghani et al.,[21] in which the partial pressures of SO2, S2, and O2 in the gas phase were controlled by Ar-SO2-S2 gas mixtures.
At silica saturation, the Al2O3 and CaO additions in iron silicate slags led to an increase of the copper concentration in matte at a given \( P_{{{\text{O}}_{ 2} }} \). For example, under the condition of \( P_{{{\text{O}}_{ 2} }} \) = 10−7.36, the copper content in the matte in the Al2O3 and CaO-free system was around 66 wt pct, and it increased to approximately 68 and 70 wt pct in the Al2O3- and (Al2O3 + CaO)-containing systems, respectively. Alternatively, at the given matte grade, the \( P_{{{\text{O}}_{ 2} }} \) decreased with adding Al2O3 and CaO into slags. Similar observations regarding the effects of Al2O3 and CaO on the matte grade were also found in the literature.[27,29,31] The Al2O3 and CaO additions decrease the activity of FeO in slag at silica saturation, thus leading to an increase of copper concentration in the matte, corresponding to reactions [1] and [2] suggested by Yazawa[17] and Fallah-Mehrjardi et al.,[26] respectively.
Figure 2(b) indicates that concentration of iron in the matte decreased from approximately 14 to 2 wt pct with increasing matte grade from 55 to 75 wt pct Cu, independently from \( P_{{{\text{SO}}_{ 2} }} \) and the slag modifiers Al2O3 and CaO. The present results for the iron concentration in matte had small deviations from the results by other researchers,[9,11,13,25,26,31] but they conform the overall decreasing trend with increasing matte grade. These investigations[9,11,13,25,26,31] had some differences compared to this study in their experimental conditions employed, such as \( P_{{{\text{SO}}_{ 2} }} \), slag composition and/or minor elements.
The sulfur concentration in the matte obtained at \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm, shown in Figure 2(c), decreased from around 23 to 20 wt pct within the matte grade range investigated. The results for sulfur concentrations in matte in the present study were identical to the observations at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm,[11,15] suggesting that the sulfur concentration in the matte was not sensitive to the prevailing SO2 partial pressure. The results in the literature[9,13,20,25,26] are on the higher side of the present results, with similar general decreasing trends.
As for the oxygen concentrations in matte obtained at \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm in Figure 2(d), decreasing with increasing copper content in matte, were likely independent of the partial pressure of SO2. The present results agreed well with the data in previous studies.[9,11,13,25] As reported by Fallah-Mehrjardi et al.,[27] the decreasing trend of oxygen concentration in matte may be related to a stronger ability of iron to form an oxy-sulfide liquid solution at low matte grades. The Al2O3 and CaO additions had no quantifiable effect on the oxygen concentration in the matte, which corresponds to the observations made for iron and sulfur concentrations.[13,15,27,29,31]
Slag Composition
The slag compositions with their standard deviations as error bars against matte grade or silica concentration are shown in Figure 3. The results from the literature[11,13,16,20,21,25,26,31] were also plotted in the graphs for comparison. Figure 3(a) shows that the copper concentration in FeOx-SiO2 slag at tridymite saturation at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm stayed almost constant up to the matte grade of 65 wt pct Cu, and then it started to increase with increasing matte grade. Similar observations for copper solubility in slags were also reported in the literature.[11,13,19,20,21,23] However, when Al2O3 and CaO were added in the slag, the copper loss in slags started to increase earlier, at lower matte grades. It also appeared that copper loss in the slag was not sensitive to the partial pressure of SO2 at a given matte grade, which corresponds to some previous observations.[18,19,20,21,22,26,39] However, some researchers[40,41] reported that the copper content in the slag increased significantly with increasing \( P_{{{\text{SO}}_{ 2} }} \).
The copper concentration in the slags generally decreased with the addition of Al2O3 and CaO. The simultaneous addition of Al2O3 and CaO decreased the copper in slag by approximately 0.4 wt pct over the entire matte grade range investigated. This positive impact of Al2O3 and CaO on decreasing copper loss in the slags can be also found in other studies.[13,29,31,42,43,44,45,46,47] However, the increasing order of Al2O3 and CaO on lowering the copper loss in slags observed in the present work is contrary to the results by Shimpo et al.,[48] in which the phase equilibrium between copper matte and FeOx-SiO2-based slag was studied at metallic copper and silica double saturation. They reported that the concentration of copper in the CaO-containing slags was higher than that in the Al2O3-containing slags when the matte grade was < 60 wt pct Cu, after which the CaO-containing slag exhibited a lower copper solubility. It seems that their results are contradictory to other literature. The small additions of basic oxides such as CaO and MgO break up the silica polymers, thus lowering the melting point and viscosity of the slag,[49,50] resulting in a decrease of copper losses due to physical entrainment in slags. The chemical losses decreased because of the replacement of copper cations by Al3+ and Ca2+.[49] The results for the copper concentration in slags provide potential for improving the recovery of the copper value from slags by controlling the matte grade and adding basic oxides into the smelting system.
Figure 3(b) indicates that the sulfur concentration in all slags decreased with increasing matte grade, similar to the trends described in previous studies.[13,20,31] The pure FeOx-SiO2 slags exhibited the highest sulfur solubilities, and it was remarkably reduced by Al2O3 and CaO additions.[13,27,29,31] The Al2O3 addition decreased the sulfur concentration by about 50 pct compared to the Al2O3-free slag at the matte grade of 65 wt pct Cu. The additional CaO in slag led to a further reduction of sulfur content in slag at the given matte grade. The impact of Al2O3 and CaO on decreasing the sulfur content in slag decreased at higher matte grades towards 75 wt pct Cu. Somewhat surprisingly, there seemed to be no strong correlation between the sulfur concentration in the slag and \( P_{{{\text{SO}}_{ 2} }} \).
Iron is present as Fe2+ and Fe3+ in the slags under the conditions of the present study. As the valence state of iron cannot be measured by EPMA, for ease of presentation in the present study, the total iron content in the silica-saturated iron silicate slags was recalculated to “FeO,” as shown in Figure 3(c). The “FeO” concentration in the silica-saturated, pure FeOx-SiO2 slag had a slightly decreasing trend with increasing matte grade, as reported by Sukhomlinov et al.[13] and Fallah-Mehrjardi et al.[25,26] With the slag modifiers Al2O3 and CaO, the “FeO” concentration in the silica-saturated FeOx-SiO2-Al2O3 and FeOx-SiO2-Al2O3-CaO slag stayed almost constant at 46 and 31 wt pct, respectively. The results in the Al2O3 and CaO-containing slags have similar trends as reported by Sukhomlinov et al.[13] but the alumina and lime concentrations were different. A comparison of the two data sets obtained at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 and 0.5 atm shows that the “FeO” concentration in slag is independent of the \( P_{{{\text{SO}}_{ 2} }} \).
The SiO2 concentrations in slags, shown in Figure 3(d), increased remarkably by approximately 5 wt pct with increasing \( P_{{{\text{SO}}_{ 2} }} \), similar to the observations by Sun et al.[35] The present results for all slags had similar trends with the results obtained at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm.[13,15] The SiO2 concentration in the FeOx-SiO2 slag increased slightly from 37 to 40 wt pct over the matte grade range studied, but the results with Al2O3- and CaO-containing slags showed constant values as a function of matte grade. However, in the study by Roghani et al.[21] at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 to 1 atm, the SiO2 concentration in the FeOx-SiO2-MgO slag was reported to decrease with increasing matte grade within the matte grade range studied in the present work. They reported that the decrease may be ascribed to the increasing copper solubility in the slag at higher matte grades, and solid silica inclusions floating on top of the slag layer, as well as solid olivine formed as a consequence of using MgO crucibles, may have been a source of error in the silica analyses.[21]
The Fe/SiO2 ratio in slag is a crucial parameter in pyrometallurgical copper smelting, as it is strongly related to the viscosity and liquidus temperature of slag. Figure 3(e) shows that the Fe/SiO2 ratio in the pure FeOx-SiO2 slag decreased slightly with increasing matte grade, as reported in previous studies,[13,15,16,25,26] whereas the results for the Al2O3-containing and CaO-containing slags exhibited no evident changes,[13,15] indicating that the addition of Al2O3 and CaO had little effect on the Fe/SiO2 ratio in the present study. The slight changes in silica and total iron content in slags contributed to the limited effect of matte grade on Fe/SiO2 ratio in this study. The overall increase of SiO2 concentration in slag compared with the results at 0.1 atm of \( P_{{{\text{SO}}_{ 2} }} \) lead to the decrease of Fe/SiO2 ratio in slag at \( P_{{{\text{SO}}_{ 2} }} \) = 0.5 atm. A similar decrease of the Fe/SiO2 ratio in slag by increasing \( P_{{{\text{SO}}_{ 2} }} \) from 0.1 to 0.25 atm at 1200°C was also reported by Fallah-Mehrjardi et al.[16,25]
The experimentally measured Al2O3 and CaO concentrations in the silica-saturated slags were plotted as a function of SiO2 content and are shown in Figures 3(f) and (g), respectively. Both the Al2O3 and CaO concentrations in the present study increased with increasing SiO2 concentration in slags for each slag series, as reported in our previous studies at \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm.[13,15] This indicates that the Al2O3 and CaO additions increase the solubility of silica in iron silicate slags,[51] although the Al2O3 and CaO concentrations were different between the compared studies. Therefore, when the Al2O3 and CaO concentrations in the silica-saturated FeOx-SiO2-based slag increased, more SiO2 should be added at the fixed conditions for attaining fully molten slag and avoiding high content of solids in the smelting slag.
The experimentally measured slag compositions (tridymite-slag phase boundaries) at 0.1 and 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \) were plotted onto the superimposed phase diagrams of FeOx-SiO2-Al2O3 and FeOx-SiO2-Al2O3-CaO systems, shown in Figures 4 and 5, respectively. The phase diagrams were calculated by MTDATA thermodynamic software using the MTOX database.[36] The prevailing \( P_{{{\text{O}}_{ 2} }} \) for Figures 4 and 5 is 10−7.8 and 10−7.3 atm, respectively, which are the presenting middle points of the oxygen partial pressure ranges investigated at each \( P_{{{\text{SO}}_{ 2} }} \). Our previous experimental results achieved at 1300 °C and 0.1 atm \( P_{{{\text{SO}}_{ 2} }} \)[15] fit well with the calculated phase boundaries in Figure 4, whereas the present experimental data at 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \) are on the higher side of the predicted lines, shifting the tridymite-slag phase boundary towards the SiO2 corner, as shown in Figure 5. The increased SiO2 concentration with increasing \( P_{{{\text{SO}}_{ 2} }} \), as shown in Figure 3(d), is the main reason for the difference between the experimental results at 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \) and the predicted computational lines. It should be noted that modeling was executed for ‘pure’ FeOx-SiO2-Al2O3(-CaO) systems by varying \( P_{{{\text{O}}_{ 2} }} \), whereas the experiments in the present study included additional elements, such as S, Cu, and other minor elements. This is one of the main reasons for the deviations between the experimental and computational results. Moreover, it is evident that the \( P_{{{\text{SO}}_{ 2} }} \) had a great impact on the slag composition, and the experimental results were not possible to replicate with MTDATA by varying only \( P_{{{\text{O}}_{ 2} }} \). Although deviations existed, the calculated phase diagrams give guidance for exploring the primary phases and phase assemblages of slags.
An isothermal section of the superimposed phase diagrams of FeOx-SiO2-Al2O3 (solid lines) and FeOx-SiO2-Al2O3-8 wt pct CaO (dotted lines) systems at 1300 °C and \( P_{{{\text{O}}_{ 2} }} \) = 10−7.8 atm with the plotted experimental data.[15] OX_LIQ-molten slag; G-gas; TRI-tridymite; SP-spinel; FSP-feldspar; MUL-mullite; ⊡CaO-free slags at 0.1 atm \( P_{{{\text{SO}}_{ 2} }} \); ⊙CaO-containing slags at 0.1 atm \( P_{{{\text{SO}}_{ 2} }} \)
An isothermal section of the superimposed phase diagrams of FeOx-SiO2-Al2O3 (solid lines) and FeOx-SiO2-Al2O3-8 wt pct CaO (dotted lines) systems at 1300 °C and \( P_{{{\text{O}}_{ 2} }} \) = 10−7.3 atm with the plotted experimental data. OX_LIQ refers to molten slag; G refers to gas; TRI refers to tridymite; SP refers to spinel; FSP refers to feldspar; MUL refers to mullite; △CaO-free slags of this study at 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \); △CaO-containing slags of this study at 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \)
Distributions of Copper, Iron, and Sulfur Between Matte and Slag
The deportment of valuable and impurity elements into the matte phase can be described by the elemental matte-slag distribution coefficients. The distribution coefficients of copper, iron, and sulfur between matte and slag were calculated using the following Eq. [3]:
where [wt pct Me] and (wt pct Me) refer to the experimentally measured elemental concentrations in matte and slag, respectively. The distribution coefficients of elements investigated are displayed in Figure 6 as a function of matte grade.
The distribution coefficients for copper between matte and FeOx-SiO2 slag had no significant changes over the entire matte grade range studied, fluctuating around 60. The values for Al2O3- and CaO-containing slag were higher and decreased along with increasing matte grade,[13,15] indicating that lower matte grade and additions of Al2O3 and CaO were favored regarding the deportment of copper into the matte phase. The copper distribution coefficients seemed not to be affected by the \( P_{{{\text{SO}}_{ 2} }} \), similarly to the distribution coefficients of iron and sulfur. The distribution coefficient values for iron were < 1, suggesting that iron was highly distributed into the slag and its deportment into slag was improved by increasing the matte grade. Contrary to the results of copper and iron, the distribution coefficients of sulfur had an increasing trend with increasing matte grade.[13] The distribution coefficient of sulfur between matte and pure FeOx-SiO2 slag was around 25 at the matte grade of 65 wt pct Cu, and its value increased to approximately 60 and 150 by Al2O3 and (Al2O3 + CaO) additions, respectively.
Conclusions
The present study provides new fundamental information for the experimental phase equilibria of copper mattes and different silica-saturated FeOx-SiO2, FeOx-SiO2-Al2O3, and FeOx-SiO2-Al2O3-CaO slags under the conditions of 1300 °C and 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \). The current observations involved high-temperature equilibration in silica crucibles under controlled CO-CO2-SO2-Ar flowing gas atmosphere, quenching of the equilibrated samples, and direct phase analyses by EPMA. The present results will not only deepen our understanding on the equilibration of matte/slag/tridymite system but can also provide new insight and guidance for the industrial operation practices.
A comparison of the data obtained at 0.1 and 0.5 atm \( P_{{{\text{SO}}_{ 2} }} \) indicates that concentrations of iron and sulfur in the matte were independent of \( P_{{{\text{SO}}_{ 2} }} \) and additions of Al2O3 and CaO at a given matte grade. However, by increasing the \( P_{{{\text{SO}}_{ 2} }} \), a higher \( P_{{{\text{O}}_{ 2} }} \) is needed to obtain a given matte grade. Copper loss in the slag increased with increasing matte grade but did not vary with \( P_{{{\text{SO}}_{ 2} }} \). Al2O3 and CaO additions into iron silicate slags can effectively decrease copper losses in the slag. Sulfur concentration in the slag decreased with increasing matte grade and with Al2O3 and CaO additions, but it was not sensitive to \( P_{{{\text{SO}}_{ 2} }} \). The equilibrium SiO2 concentration in silica-saturated iron silicate slags generally increased by 5 to 7 wt pct with increasing \( P_{{{\text{SO}}_{ 2} }} \), and the corresponding Fe/SiO2 ratio of the slags was lower at higher \( P_{{{\text{SO}}_{ 2} }} \), i.e., 0.5 atm. The increase of Al2O3 and CaO concentrations in silica-saturated iron silicate slags also led to an increase of SiO2 solubility in the slags.[51]
References
P. Taskinen, K. Seppälä, J. Laulumaa, and J. Poijärvi: Miner. Process. Extr. Metall., 2001, vol. 110 (2), pp. 94-100.
I. V. Kojo, A. Jokilaakso, and P. Hanniala: JOM, 2000, vol. 52 (2), pp. 57-61.
P. Taskinen, A. Jokilaakso, D. Lindberg, and J. Xia: Miner. Process. Extr. Metall., 2020, 129:2017-220. https://doi.org/10.1080/25726641.2019.1688904
P. Taskinen: AIP Conference Proceedings, AIP Publishing LLC, 2017, vol. 1805, No. 1, pp. 020001. 10.1063/1.4974407.
P. Taskinen: Miner. Process. Extr. Metall., 2011, vol. 120 (4), pp. 240-246. https://doi.org/10.1179/1743285511Y.0000000013
I.V. Kojo and H. Storch: Sohn International Symposium, Warrendale PA, TMS, 2006, vol. 8, pp. 225–38.
M. E. Schlesinger, K. C. Sole, and W. G. Davenport: Extractive metallurgy of copper. 5th ed., Elsevier, Oxford, 2011
R. Sridhar, J. M. Toguri, and S. Simeonov: JOM, 1997, vol. 49(4), p. 48.
Y. Takeda: Molten Slags, Fluxes and Salts’ 97 Conference, Sydney, Iron & Steel Society, Warrendale, PA, 1997, pp. 329–39.
K. Yamaguchi: Extraction 2018, Davis B. et al. (eds), Ottawa, Springer, Cham, 2018, pp. 797–804. https://doi.org/10.1007/978-3-319-95022-8_63.
K. Avarmaa, H. Johto, and P. Taskinen: Metall. Mater. Trans. B, 2016, vol. 47B (1), pp. 244-55.
K. Avarmaa, H. O’Brien, H. Johto, and P. Taskinen: J. Sustain. Metall., 2015, vol. 1 (3), pp. 216-28.
D. Sukhomlinov, L. Klemettinen, H. O’Brien, P. Taskinen, and A. Jokilaakso: Metall. Mater. Trans. B, 2019, vol. 50B (6), pp. 2723-32.
M. Chen, K. Avarmaa, L. Klemettinen, H. O’Brien, D. Sukhomlinov, J. Shi, P. Taskinen, and A. Jokilaakso: Recovery of precious metals (Au, Ag, Pt, and Pd) from urban mining through copper smelting. Metall. Mater. Trans. B, 2020, vol. 51B (4), pp. 1495–508. https://doi.org/10.1007/s11663-020-01861-5.
M. Chen, K. Avarmaa, L. Klemettinen, J. Shi, P. Taskinen, and A. Jokilaakso: Experimental study on the phase equilibrium of copper matte and silica-saturated FeOx-SiO2-based slags in pyrometallurgical WEEE processing. Metall. Mater. Trans. B, 2020, vol. 51B (4), pp. 1552–63. https://doi.org/10.1007/s11663-020-01874-0
A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Int. J. Mater. Res., 2019, vol. 110 (6), pp. 489-95.
A. Yazawa: Can. Metall. Q., 1974, vol. 13 (3), pp. 443-53.
F. J. Tavera and W. G. Davenport: Metall. Trans. B, 1979, vol. 10B (2), pp. 237-41.
G. Roghani, M. Hino, and K. Itagaki: Proceedings of 5th International Conference on Molten Slags, Fluxes and Salts. Iron & Steel Society, Warrendale, 1997, pp. 693–703.
G. Roghani, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1997, vol. 38 (8), 707-13.
G. Roghani, Y. Takeda, and K. Itagaki: Metall. Mater. Trans. B, 2000, vol. 31B (4), pp. 705-12.
G. Roghani, J. C. Font, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1996, vol. 37 (10), pp. 1574-79.
Y. Takeda: Proceedings of 5th International Conference on Molten Slags, Fluxes and Salts. Iron & Steel Society, Warrendale, 1997, pp. 735–43.
A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2017, vol. 48B (6), pp. 3002-16.
A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2017, vol. 48B (6), pp. 3017-26.
A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (4), pp. 1732-39.
A. Fallah-Mehrjardi, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (2), pp. 602-09.
H. Abdeyazdan, A. Fallah-Mehrjardi, T. Hidayat, M. Shevchenko, P. C. Hayes, and E. Jak: J. Phase Equilib. Diffus., 2020, vol. 41 (1), pp. 44-55.
H. Abdeyazdan, A. Fallah-Mehrjardi, T. Hidayat, M. Shevchenko, P. C. Hayes, and E. Jak: J. Phase Equilib. Diffus., 2020, vol. 41 (1), pp. 66-78.
D. Shishin, E. Jak, and S. A. Decterov: J. Phase Equilib. Diffus., 2018, vol. 39 (5), pp. 456-75.
D. Shishin, T. Hidayat, A. Fallah-Mehrjardi, P. C. Hayes, S. A. Decterov, and E. Jak: J. Phase Equilib. Diffus., 2019, vol. 40 (4), pp. 445-61.
T. Hidayat, A. Fallah-Mehrjardi, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (4), pp. 1750-65.
T. Hidayat, A. Fallah-Mehrjardi, P. C. Hayes, and E. Jak: Advances in Molten Slags, Fluxes, and Salts-Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts. Springer, Cham, 2016, pp. 1207–20.
M. Chen, Y. Sun, E. Balladares, C. Pizarro, and B. Zhao: Calphad, 2019, vol. 66, pp. 101642.
Y. Sun, M. Chen, E. Balladares, C. Pizarro, L. Contreras, and B. Zhao: Calphad, 2020, vol. 69, pp. 101751.
J. Gisby, P. Taskinen, J. Pihlasalo, Z. Li, M. Tyrer, J. Pearce, K. Avarmaa, P. Björklund, H. Davies, M. Korpi, S. Martin, L. Pesonen, and J. Robinson: Metall. Mater. Trans. B, 2017, vol. 48B (1), pp. 91-98.
SGTE Database for Pure Substances, Scientific Group Thermodata Europe. http://www.sgte.org/.
J.L. Pouchou and F. Pichoir: 11th International Congress on X-Ray optics and Microanalysis (ICXOM), J.D. Brown and R.H. Packwood, eds. Ontario, Canada, 1986, pp. 249–56.
G. Roghani, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1996, vol. 37 (8), pp. 1431-37.
A. Yazawa and M. Eguchi: Extractive Metallurgy of Copper, J.C. Yannopoulos and J.C. Agarwal, eds. AIME, New York, 1976, vol.1, pp. 3–20.
F. Sehnalek and I. Imris: Advances in Extractive Metallurgy and Refining, M.J. Jones, ed. Inst. Mining Metal., London, 1972, pp. 39–62.
H. G. Kim and H. Y. Sohn: Metall. Mater. Trans. B, 1998, vol. 29B (3), pp. 583-90.
L. Klemettinen, K. Avarmaa, and P. Taskinen: J. Sustain. Metall., 2017, vol. 3 (4), pp. 772-81.
K. Avarmaa, S. Yliaho, and P. Taskinen: Waste Manage., 2018, vol. 71, pp. 400-10.
K. Avarmaa, H. O’Brien, L. Klemettinen, and P. Taskinen: J. Mater. Cycles Waste Manage., 2019, https://doi.org/10.1007/s10163-019-00955-w
K. Avarmaa, L. Klemettinen, H. O’Brien, and P. Taskinen: Miner. Eng., 2019, vol. 133, pp. 95-102.
M. Nagamori, P. J. Mackey, and P. Tarassoff: Metall. Trans. B, 1975, vol. 6B (2), pp. 295-301.
R. Shimpo, S. Goto, O. Ogawa, and I. Asakura: Can. Metall. Q., 1986, vol. 25 (2), pp. 113-21.
P. J. Mackey: Can. Metall. Q., 1982, vol. 21 (3), pp. 221-60.
B. Keyworth: 6th International Precious Metals Institute, California, Pergamon, 1983, pp. 509–37. https://doi.org/10.1016/b978-0-08-025396-1.50044-0.
E. T. Turkdogan: Physicochemical properties of molten slags and glasses, London: The Metals Society, 1983, pp. 99-122.
Acknowledgments
This work has been financially supported by Aalto University School of Chemical Engineering and the SYMMET project by Business Finland (grant #3891/31/2018). We made use of the Academy of Finland’s RawMatTERS Finland Infrastructure (RAMI) based at Aalto University, GTK Espoo, and VTT Espoo. Mr. Lassi Pakkanen of Geological Survey of Finland (GTK) is especially appreciated for conducting the EPMA analyses. Mr. Min Chen would like to thank the China Scholarship Council (grant #201806370217) for the financial support of his study at Aalto University in Finland.
Conflict of interest
The authors declare that they have no competing interest.
Funding
Open access funding provided by Aalto University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 20, 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Avarmaa, K., Klemettinen, L. et al. Equilibrium of Copper Matte and Silica-Saturated Iron Silicate Slags at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) of 0.5 atm. Metall Mater Trans B 51, 2107–2118 (2020). https://doi.org/10.1007/s11663-020-01933-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01933-6